Study tests ‘pre-fertilization’ in nursery phase of a biofloc system

In aquaculture, successful production depends on favorable conditions – both physical and chemical – for the growth of the target species. Water quality maintenance plays an important role in providing a suitable environment for cultured animals, especially in increasingly intensive technical culture systems. Systems that use biofloc technology combine a number of advantages to get better performance from cultured marine shrimp. One of these advantages is improved water quality achieved by recycling nutrients derived from nitrogen, such as ammonia and nitrite. By keeping concentrations of the nitrogenous compounds below toxic levels, the different phases of shrimp farming have better results.
In this sense, the nursery phase may be used for biofloc systems prior to animal stocking for grow-out. Size uniformity in animals and reducing the risk of diseases are the main advantages of nurseries. As a short-term phase, it can be used to start the biofloc formation and water reuse in the grow-out phase.
In biofloc systems for marine shrimp culture, the main source of nitrogen is the feed offered. Cultured organisms metabolize the feed and excrete nitrogen as ammonia. Another source of ammonia in the water is the decomposition of feed not consumed by bacteria. The control of ammonia levels is separated into three processes: absorption by microalgae, bacterial assimilation and nitrification.
Ammonia, bacteria in miofloc systems
The maintenance of heterotrophic and nitrifying (autotrophic) bacteria in biofloc systems depends on the dissolved ammonia in the pond water. At the beginning of the cycle, the heterotrophic bacteria are stimulated by the addition of organic carbon, then consume and metabolize ammonia, incorporating it in microbial biomass. Subsequently, the nitrifying bacteria oxidize ammonia to nitrite and to nitrate through autotrophic aerobic bacteria groups known as Nitrosomonas and Nitrobacter.
The Nitrosomonas group is responsible for ammonia oxidation to nitrite, whereas Nitrobacter oxidizes nitrite to nitrate. However, in using ammonia, autotrophic and heterotrophic bacteria mainly depend on the nutrient from cultured organisms’ excretions.
In traditional systems, the ammonia concentration tends to increase over the production cycle due to the increasing biomass of shrimp cultured, a greater supply of inert food and organic matter accumulation. Even following established recommendations for feeding, traditional semi-intensive and intensive production systems must often perform partial exchange of water so that ammonia concentrations do not affect the animals’ growth or reach lethal levels.
Minimizing ammonia accumulation

One of the ways to minimize ammonia accumulation in biofloc systems is to accelerate the nitrifying process before the entry of ammonia to the system. This addition can be made before or after shrimp stocking in order to establish the ammonium-oxidizing bacteria.
In order to provide nutrients for the biofloc formation, the frequency of the additions is important because the initial phase involves dominant heterotrophic bacteria. These bacteria have a high capacity to capture the ammonia and convert it into microorganism protein. This limits the availability of nutrients for autotrophic bacteria, which already have slow growth. As a result, nitrite, the intermediate product of nitrification, can reach undesirable levels.
Strategies for adding ammonia in different concentrations and intervals vary depending on the responses of biofloc formation due to the stimulation of microbial growth by nutrient supply. These variations occur because the addition can be made before the beginning of culture or after the stocking of animals.
It is noteworthy that the presence of shrimp affects ammonia concentration due to animal excretion and decomposition of organic matter. The ammonia levels in the culture system should therefore be considered in calculations for the addition of compounds that contain ammonia.
Ammonia study
At the Marine Aquaculture Station of the Federal University of Rio Grande in southern Brazil, experiments were conducted to assess the microbial aggregation rate in a biofloc system for Pacific white shrimp (Litopenaeus vannamei). Financial support for the work was provided by the National Council for Scientific and Technological Development, the Ministry of Fishery and Aquaculture and Coordination for the Improvement of Higher Level Personnel.
The pre-test analyzed different concentrations of ammonium chloride in the grow-out phase, with concentrations of 1.5 and 3.0 mg/L at two frequencies of application (three and seven days), compared to treatment without the ammonia addition.
Fig. 1 shows that where ammonia was added, higher amounts of settleable solids were observed compared to the treatment without the use of the nutrient. It was also found that the most frequent applications had higher amounts of biofloc settled at the end of the test. The observation that supplying nutrients (ammonia) frequently stimulated biofloc formation during culture verified the likelihood of accelerating this formation by starting the process prior to stocking animals.
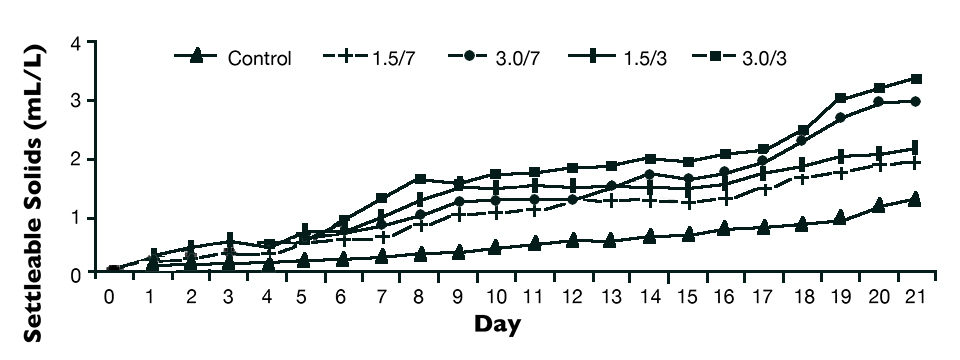
In a study, “pre-fertilization” was tested in the nursery phase. The objective was to accelerate the biofloc formation to minimize ammonia concentrations, avoiding high peaks during culture. This also encouraged the establishment of bacterial communities involved in the nitrifying process.
The experiment was conducted in a greenhouse with nine, 35-square-meter tanks, comprising three treatments with three replicates. Over a week, ammonium chloride was added at concentrations of 0.5 and 3.0 mg/L, composing two treatments. Sugar cane molasses was used as a carbon source to reach a carbon:nitrogen ratio of 6:1.
A control treatment without the addition of ammonia was used to compare the biofloc formation rate. The ammonia concentration was measured in the water culture water as the total ammonia nitrogen (TAN). Shrimp with an average weight of 0.03 grams were stocked at a density of 3,000 per square meter.
Results
The nitrogen compound concentrations did not differ significantly among treatments (Table 1). Note that even without the addition of ammonium chloride, the average TAN concentration in the control treatment was similar to the TAN values where there was nutrient addition, demonstrating the feasibility of pre-fertilization without compromising the ammonia balance in culture.
The highest total suspended solids concentrations occurred immediately after the pre-fertilization in the test treatments (Fig. 2). This demonstrated the more rapid formation of microbial aggregates with utilization of ammonium chloride.
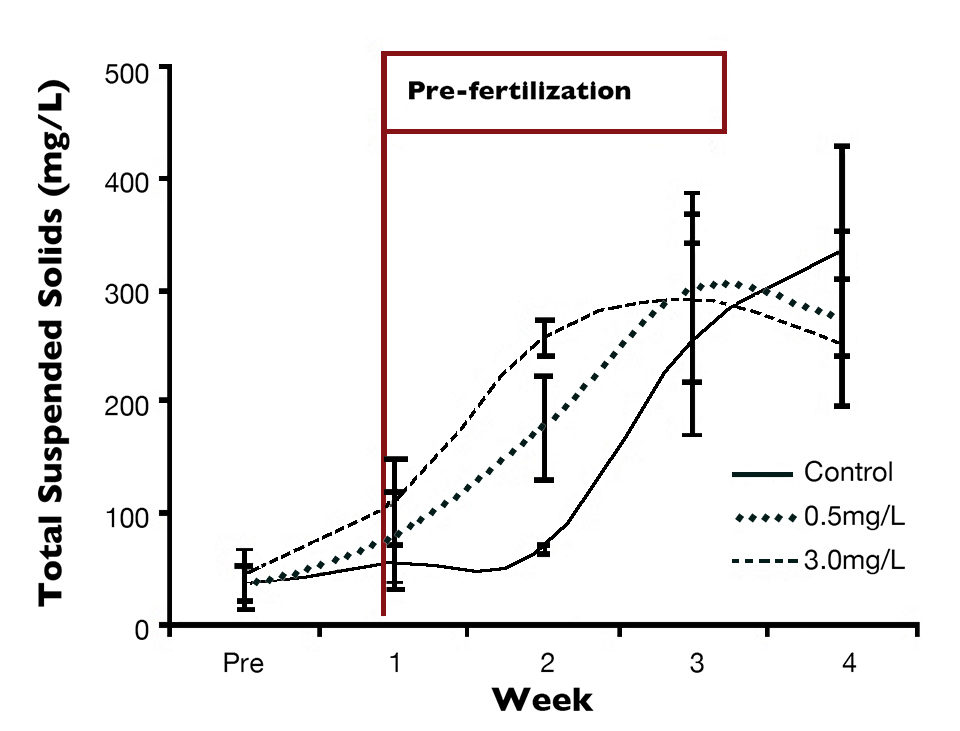
In a general way, the fertilization can be defined according to the time of culture – before stocking and making use of pre-fertilization, as well as the addition of ammonia after the animals are stocked.
The acceleration of biofloc formation in the nursery phase enables water reuse for grow-out, optimizing the maintenance of water quality. The use of ammonia for fertilizing biofloc systems is feasible and promotes the emergence of bacteria to form biofloc without compromising water quality.
(Editor’s Note: This article was originally published in the July/August 2014 print edition of the Global Aquaculture Advocate.)
Authors
-
Carlos Gaona
Ph.D. Student
Laboratório de Carcinocultura
Universidade Federal do Rio Grande
Rio Grande 96201-900 Brazil -
Dariano Krummenauer, Ph.D.
Laboratório de Carcinocultura
Universidade Federal do Rio Grande
Rio Grande 96201-900 Brazil -
André Freitas
Laboratório de Carcinocultura
Universidade Federal do Rio Grande
Rio Grande 96201-900 Brazil -
Márcio Miranda, M.S.
Laboratório de Carcinocultura
Universidade Federal do Rio Grande
Rio Grande 96201-900 Brazil -
Luis Poersch, Ph.D.
Laboratório de Carcinocultura
Universidade Federal do Rio Grande
Rio Grande 96201-900 Brazil -
Wilson Wasielesky, Jr., Ph.D.
Laboratório de Carcinocultura
Universidade Federal do Rio Grande
Rio Grande 96201-900 Brazil
Tagged With
Related Posts

Responsibility
A look at various intensive shrimp farming systems in Asia
The impact of diseases led some Asian shrimp farming countries to develop biofloc and recirculation aquaculture system (RAS) production technologies. Treating incoming water for culture operations and wastewater treatment are biosecurity measures for disease prevention and control.

Health & Welfare
Acclimating shrimp postlarvae before pond stocking
Shrimp postlarvae acclimation before stocking into the various growout systems (ponds, raceways, tanks) is a critical – and often overlooked, sometimes taken for granted – step in the shrimp culture process. Various water quality parameters should be changed slowly so that the young shrimp have the time to gradually adapt to the new conditions.

Aquafeeds
Biofloc consumption by Pacific white shrimp postlarvae
The stable isotopes technique with δ13C and δ15N can be used to determine the relevance of different food sources to shrimp feeding during the pre-nursery phase of Litopenaeus vannamei culture. During this trial, different types of commercial feed, microalgae, Artemia sp. nauplii and bioflocs were used as food sources.

Innovation & Investment
Recirculating aquaculture technology, part 1
Biological filtration is one of the critical required unit processes in recirculating aquaculture systems. Non-corrosive filter media with large amounts of surface provides area for nitrifying bacteria cells to colonize.


