Results show phenotype switching, decreased virulence and improved survival

Multiple factors might be involved in outbreaks of Acute Hepatopancreatic Necrosis Disease (AHPND) – a disease that has resulted in farmed shrimp losses exceeding an estimated U.S. $43 billion – but the presence of virulent Vibrio parahaemolyticus (VPAHPND) is the primary factor. However, a compromised health status of the cultured animals in combination with suboptimal environmental conditions most likely facilitates AHPND outbreaks, resulting in high mortalities of juvenile shrimp and often entire loss of stocks within 30 days of stocking.
Therefore, disease prevention and control measures should not only focus on maintaining a biosecure environment but should be based on an integral approach ensuring, among other measures, adequate nutrition and good health of the aquatic animals and maintenance of optimum water quality.
Shrimp production in biofloc systems increases feed utilization, growth and survival. Biofloc can also act as an immunostimulant to enhance the shrimp innate immune system and provide protection against microbial pathogens, including the AHPND-causing V. parahaemolyticus. Biofloc can decrease the impact of the AHPND V. parahaemolyticus challenge; however, its protective potential depends on operational parameters of the biofloc system. In this context, there is an urgent need to better understand the protective mechanisms of a biofloc environment and develop a biofloc system that can help control AHPND infection in shrimp aquaculture.
Based on observations of V. parahaemolyticus phenotype switching (the change or switching between multiple cellular morphologies) in altered environmental conditions, and results reported by other researchers on the protective potential of biofloc system against AHPND-causing V. parahaemolyticus, we hypothesized that a biofloc environment is involved in setting the phenotypic status of AHPND-causing V. parahaemolyticus under both in vitro and in vivo conditions, thus altering its virulence. We tested this hypothesis using a biofloc-based model for V. parahaemolyticus and Pacific white shrimp (Litopenaeus vannamei) postlarvae, or PLs.
This article – adapted and summarized from the original (Kumar V, Wille M, Lourenço TM and Bossier P. 2020. Biofloc-based enhanced survival of Litopenaeus vannamei upon AHPND-causing Vibrio parahaemolyticus challenge is partially mediated by reduced expression of its virulence genes. Front. Microbiol. 11:1270. doi:10.3389/fmicb.2020.01270) – reports on a study to evaluate the potential of a biofloc environment to alter the virulence of an AHPND-causing V. parahaemolyticus strain on L. vannamei PLs.
Study setup
The experiments were carried out at the Laboratory of Aquaculture & Artemia Reference Center, Ghent University, Belgium, using 20-liter glass aquaria tanks filled with seawater (30 grams per liter salinity) as the experimental culture units. The photoperiod was maintained with a regime of 12 hours of light and 12 hours of darkness, and the water temperature was maintained at 27.5 to 28.5 degrees-C in a controlled-temperature room.
L. vannamei PLs (initial average body weight (0.31 ± 0.02 grams) from a commercial supplier (Shrimp Improvement Systems, SIS, Florida, United States) – previously conditioned and acclimated to the experimental conditions for one week – were randomly distributed in the tanks at a density of 30 animals per tank (366 shrimps per square meter). All shrimp were fed four times daily with a commercial pellet diet (CreveTec Grower 2, Belgium) with 35 percent crude protein and 10 percent crude fat for 28 days. The feeding amounts were estimated based on 7 percent animal wet body weight per day, and the daily feed amount was adjusted to the biomass in the tanks.
For biofloc development, a biofloc starter culture from an established biofilter system was used to seed the treatment and control tanks. During the 28-days experimental period, glucose (carbon source) was added daily to support the biofloc in the treatment group. Sixty percent of the culture water in the control group was exchanged daily, while in the treatment group seawater was regularly added only to make up for the water lost due to evaporation.
To determine the protective effect of biofloc for L. vannamei PLs, a challenge assay was performed with four separate shrimp groups (Fig. 1). Shrimp PLs were cultured in either the biofloc system or the clear water system for 28 days and were then transferred to 2-liter plastic containers. In the first and second group, 30 shrimp PLs from the clear water control group were collected and groups of 5 PLs per replicate were transferred to 2-liter plastic container filled with either 1 liter of clear seawater (SW) or 1 liter of biofloc water (BFW). Similarly, in the third and fourth groups, the 30 shrimp PLs collected from the biofloc system were transferred to 2-liter plastic containers and filled with either 1 liter of SW or BFW. All containers had aeration and were maintained at 27.5 to 28.5 degrees-C in a controlled-temperature room.
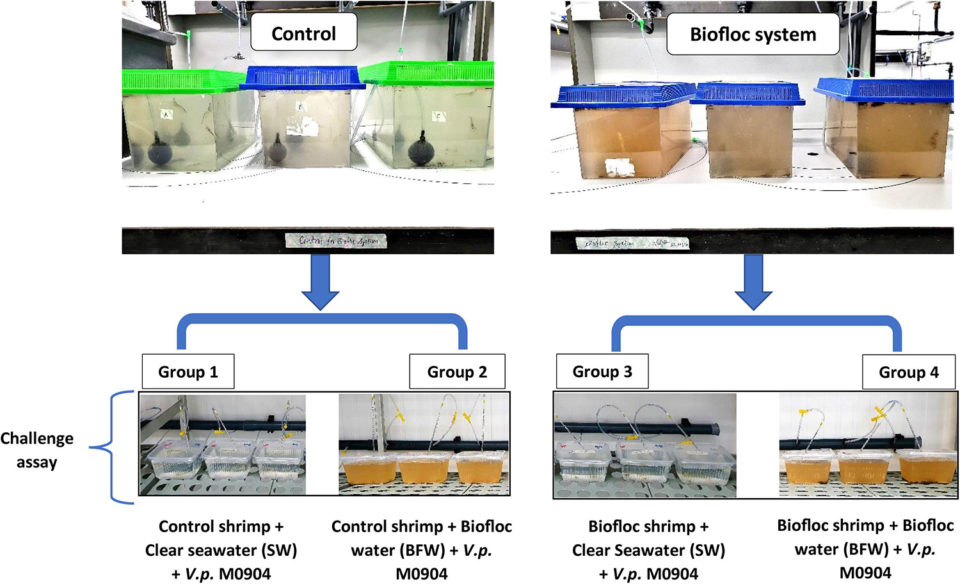
After addition of BFW, PLs were challenged by immersion with the V. parahaemolyticusM0904 strain (BCCM accession number – LMG P-31518) at 106 cells per ml. The survival of shrimp PLs was scored at 12, 24, 36 and 48 hours after the addition of the pathogen. PLs that were not challenged with V. parahaemolyticus were maintained as negative controls, and each group in the treatment and control were maintained in triplicate.
For detailed information on the experimental design; water quality monitoring; growth performance of shrimp postlarvae; challenge assay; RNA extractions and PCR analyses; and statistical analyses, refer to the original publication.
Results and discussion
The results of this study demonstrated that a biofloc system maintained at a carbon-nitrogen ratio of 15:1 enhances the survival of L. vannamei when challenged with a V. parahaemolyticus AHPND strain. These results highlight that in the biofloc system, AHPND-causing V. parahaemolyticus possibly switches from a free-living, virulent, planktonic phenotype to a non-virulent, biofilm phenotype, as demonstrated by a decreased transcription of several genes, including those involved in the production of toxins, and the increased expression of a phenotype-switching gene in both in vitro and in vivo conditions. The AHPND V. parahaemolyticus strain evaluated switches to a phenotype that is also less virulent to shrimp species. Research also showed that biofloc stimulates the innate immunity and decreases the impact of AHPND V. parahaemolyticus challenge in shrimp.
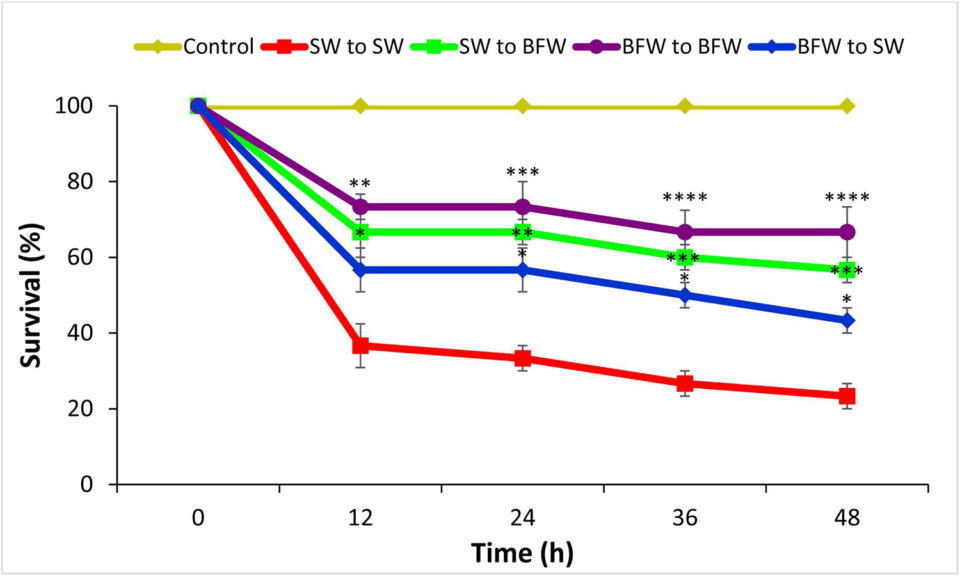
The biofloc system significantly improved the growth performance and tolerance against the AHPND-causing V. parahaemolyticus strain by L. vannamei PLs. The biofloc system is generally formed by aggregation of microorganism, microalgae, organic particles, or solids from uneaten feeds. For shrimp in a biofloc system, flocs consumed may constitute up to 29 percent of the daily feed intake. Therefore, the consumed biofloc might nutritionally modulate the shrimp health status, resulting in improved growth performance and increased protection against the V. parahaemolyticus AHPND strain tested.
Sheer stress or flow behavior (bacterial movement) is an important factor modulating the growth of bacteria, either in a free-living planktonic form or in matrix-enclosed communities (e.g., a biofilm). Hence, to gain insight into the V. parahaemolyticus phenotype status in a biofloc system, we used the qPCR technique [a real-time polymerase chain reaction, also known as quantitative polymerase chain reaction (qPCR), a laboratory technique of molecular biology based on the polymerase chain reaction, PCR that monitors the amplification of a targeted DNA molecule during the PCR, not at its end, as in conventional PCR] to assess the in vitro temporal expression of various genes, including VPAHPND virulent genes. The results indicated that expression of several genes was significantly decreased in the biofloc group when compared with the control SW group.
Because there is a correlation between flagellar (helical filamentous organelle used by bacteria to move) motility and the virulence of bacterial pathogens, the temporal downregulation (process by which a cell decreases the quantity of a cellular component, such as RNA or protein, in response to an external stimulus) of flagellar motility genes in V. parahaemolyticus suggests a decrease in its virulence in biofloc conditions.
In addition, the transcription (the process of copying a segment of DNA into RNA) of other genes, including PirBVP (responsible for AHPND’s PirBVP toxin) and others that mediate the virulence of V. parahaemolyticus was also significantly downregulated in the biofloc group, compared with the control SW group. These results suggest that V. parahaemolyticus in biofloc systems switch to a non-virulent phenotype, as deduced from the increased expression of the phenotype switching marker gene, with a simultaneous decrease of the expression of flagella-related motility and other virulent genes. This expression profile has previously been shown to be associated with the non-virulent phenotypic status of this V. parahaemolyticus AHPND strain (M0904 strain).
The shrimp hepatopancreas (HP) is considered the primary target tissue of AHPND bacteria, and we used it to study the in vivo effect of biofloc supplementation on the phenotype status and virulence of AHPND bacteria. Results showed that in the control, clear SW group, V. parahaemolyticus produce AHPND toxin in the hepatopancreas, and hence appear to adopt the planktonic mode of growth. However, in the treatment biofloc group, the expression of motility and toxin genes in the hepatopancreas were significantly downregulated.
Overall, our results suggest that biofloc indeed induces phenotype switching in V. parahaemolyticus in both in vitro and in vivo conditions, which results in increased resistance of L. vannamei against the V. parahaemolyticus AHPND strain tested. We recommend further studies on the mechanisms by which biofloc interferes with both host nutrition, and immunological response and expression of virulence genes in V. parahaemolyticus to better understand how biofloc systems enhance the survival of L. vannamei when challenged with V. parahaemolyticus AHPND.
Perspectives
The results of our study provide new insight on the protective action of biofloc systems against the V. parahaemolyticus AHPND strain evaluated. These results suggest that the protective effect of the biofloc system is at least partially caused by steering phenotype switching where AHPND-causing V. parahaemolyticus might change to a non-virulent, matrix-enclosed, biofilm phenotype.
The ability of the biofloc system to boost the water quality, growth performance and resistance of L. vannamei against the V. parahaemolyticus AHPND strain tested makes it a potent aquaculture technology valuable to prevent microbial infections, including by AHPND, and support increasing high-density shrimp production with minimal or no water exchange.
References available in the original publication.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Vikash Kumar, Ph.D.
Corresponding author
Lab of Aquaculture & Artemia Reference Center
Department of Animal Sciences and Aquatic Ecology
Faculty of Bioscience Engineering
Ghent University, Ghent, Belgium; and
ICAR-Central Inland Fisheries Research Institute (CIFRI)
Barrackpore, India -
Mathieu Wille, M.S.
Lab of Aquaculture & Artemia Reference Center
Department of Animal Sciences and Aquatic Ecology
Faculty of Bioscience Engineering
Ghent University, Ghent, Belgium -
Tânia Margarida Lourenço, M.S.
Ph.D. Student
Lab of Aquaculture & Artemia Reference Center
Department of Animal Sciences and Aquatic Ecology
Faculty of Bioscience Engineering
Ghent University, Ghent, Belgium -
Peter Bossier, Ph.D.
Lab of Aquaculture & Artemia Reference Center
Department of Animal Sciences and Aquatic Ecology
Faculty of Bioscience Engineering
Ghent University, Ghent, Belgium
Tagged With
Related Posts

Health & Welfare
Bacterial pathogens in biofilms pose health risks in recirculating systems
Recirculating aquaculture presents an increased potential for pathogenic bacteria to become established in the system through the formation of biofilms.

Health & Welfare
Caveat emptor when seeking for EMS solutions
The development of polymerase chain reaction testing to detect the bacteria that cause EMS is important, but confirmation by bioassay of presumptive positives to ensure pathogenicity is a prudent intermediate step.

Health & Welfare
Four AHPND strains identified on Latin American shrimp farms
Two virulence genes are known to encode a binary photorhabdus insect-related toxin that causes acute hepatopancreatic necrosis disease in shrimp. The pathogenicities of these V. campbellii strains were evaluated through laboratory infection and subsequent histological examination in P. vannamei shrimp.

Health & Welfare
TSV challenges advance selective breeding in Pacific white shrimp
Results of ongoing studies demonstrate that selective breeding for both resistance to Taura Syndrome Virus (TSV) and fast growth in shrimp is achievable.


