Study finds that novel Vibrio parahaemolyticus is causative agent of the emerging disease TPD

Aquaculture, including shrimp farming, is the fastest-growing sector producing food of animal origin in the world. Several emerging diseases – including acute hepatopancreatic necrosis disease (AHPND), viral covert mortality disease (VCMD), infection by Enterocytozoon hepatopenaei (EHP) and infection of shrimp hemocyte iridescent virus (SHIV) – have impacted on the global shrimp aquaculture industry. More recently, a novel disease called “translucent postlarvae disease” (TPD) or “glass postlarvae disease” (GPD) in Pacific white shrimp (Litopenaeus vannamei) has become a rapidly growing threat to shrimp farming in China recently.
Since March 2020, numerous cases of TPD have occurred in some L. vannamei hatcheries in the Provinces of Guangdong and Guangxi provinces, after which this new disease began to spread to major shrimp farming areas in the north of China via postlarvae (PL or PLs) transportation in April 2020. Translucent postlarvae disease mostly affects PLs at four to seven days old (PL4 to PL7) with very severe infectivity. Usually, the morbidity of a diseased population can reach up to 60 percent on the second day after first observing abnormal individuals, and even up to 90 to 100 percent in severe cases on the third day. Translucent postlarvae disease affected shrimp mainly show similar gross clinical signs, such as a pale or colorless hepatopancreas and empty digestive tract, which causes the body of diseased individuals to become transparent and translucent; therefore, these diseased individuals were named “translucent post-larvae” or “glass post-larvae” by local farmers.
TPD disease has become prevalent in farmed shrimp stocks, leading to serious economic losses in some shrimp farming areas in China, so it was urgent to investigate and develop strategies for preventing the disease. We sampled TPD-affected shrimp and screened for several known pathogens of shrimp, but our results indicated that these shrimp were free of known shrimp viral pathogens, and suggested that TPD might be caused by a new emerging pathogen. In addition, some farmers found that treatment of water in rearing tanks with an antibacterial agent could alleviate the disease, suggesting TPD might be caused by a bacterial pathogen.
This article – adapted and summarized from the original publication [Zou, Y. et al. 2020. Determination of the Infectious Agent of Translucent Post-Larva Disease (TPD) in Penaeus vannamei. Pathogens 2020, 9(9), 741] – reports on a systematic investigation of the potential infectious agent causing TPD that we conducted based on bacterial isolation and identification, challenge experiments, and histopathological analyses.
https://www.aquaculturealliance.org/advocate/estimating-heritability-of-ammonia-tolerance-in-pacific-white-shrimp/
Study setup
This research was carried out by the several co-authors at two institutions in Qingdao, China: the Key Laboratory of Marine Aquaculture Disease Control, Yellow Sea Fisheries Research Institute; and the Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology.
Diseased L. vannamei PLs (PL7, body length 6 to 8 mm) were sampled from a shrimp farm in Ganyu, Jiangsu Province. These moribund animals were processed for histopathological examination and for transmission electron microscope observations. Healthy postlarvae PL3, body length 4 to 6 mm) were purchased from a shrimp farm in Weifang, Shandong Province, and reared for two days, and then used for the challenge test.
For detailed information on the shrimp sample collection and the experimental design; tests for pathogen detection and identification; analyses of virulent genes in the isolated strain; experimental challenge by immersion; histopathology; and other analyses, refer to the original publication.
Results and discussion
Over the past decade, the world shrimp farming industry has been heavily affected by several emerging and re-emerging diseases. One of those major diseases is AHPND, also known as early mortality syndrome (EMS). AHPND has spread to other shrimp farming countries since it was first reported in Vietnam and China in 2010. The global prevalence of AHPND has caused significant losses in farmed shrimp production in many countries in Asia and the Americas, and the direct global economic loss caused by AHPND has been estimated at more than (U.S.) $44 billion from 2010 to 2016 (DOI: 10.20506/rst.38.2.2999 ).
The causative agent of AHPND proved to be some unique isolates of Vibrio (VAHPND), and early on VAHPNDwas suspected to be the causative agent of TPD because the gross clinical signs of the diseased PL were similar to AHPND syndrome to some degree. However, both Polymerase Chain Reaction, PCR {method widely used to rapidly make millions to billions of copies of a specific DNA sample to study it in detail] used showed negative results for the VAHPND test. In addition, AHPND occurs approximately within 35 days after stocking of shrimp ponds, and the onset of clinical signs and mortality of AHPND start as early as 10 days post-stocking in some extreme cases. But TPD usually occurs in PL4-7, which is much earlier than when AHPND usually appears. Hence, we deduced that TPD was a novel disease different from AHPND.
The results of molecular assays showed that the diseased individuals in the TPD-affected rearing tanks of post-larvae were negative for major diseases of farmed shrimp, including White Spot Syndrome Virus (WSSV), Infectious Hypodermal and Haematopoietic Necrosis Virus (IHHNV), VAHPND, Enterocytozoon hepatopenaei (EHP), Shrimp Hemocyte Iridescent Virus (SHIV), Yellowhead Virus (YHV), Taura Syndrome Virus (TSV) and Infectious Myonecrosis Virus (IMNV).
Therefore, considering that treatment of water in the larval shrimp rearing tanks with an antibacterial agent could alleviate the disease, we first conducted bacterial pathogen isolation and identification from samples of diseased L. vannamei affected with TPD. The dominant bacteria isolate from the moribund infected shrimp were identified as V. parahaemolyticus according to various tests.
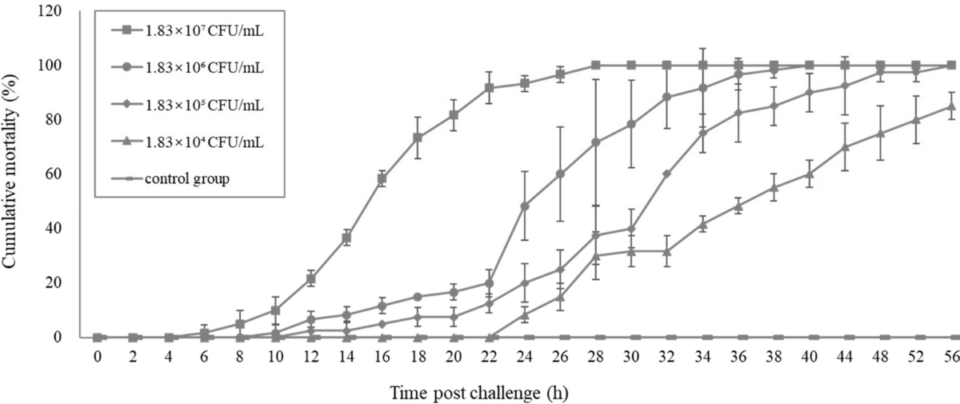
And pathogenicity analysis of these V. parahaemolyticus in a challenge test indicated that the typical gross clinical signs were similar to those of farm-raised, TPD-affected shrimp. The onset and progression of the disease in the challenge test as well as the mortality were also similar to those of farmed-based, TPD-affected shrimp. Thus, this novel V. parahaemolyticus (Vp-JS20200428004-2) was deduced to be the pathogenic agent of TPD in shrimp postlarvae.
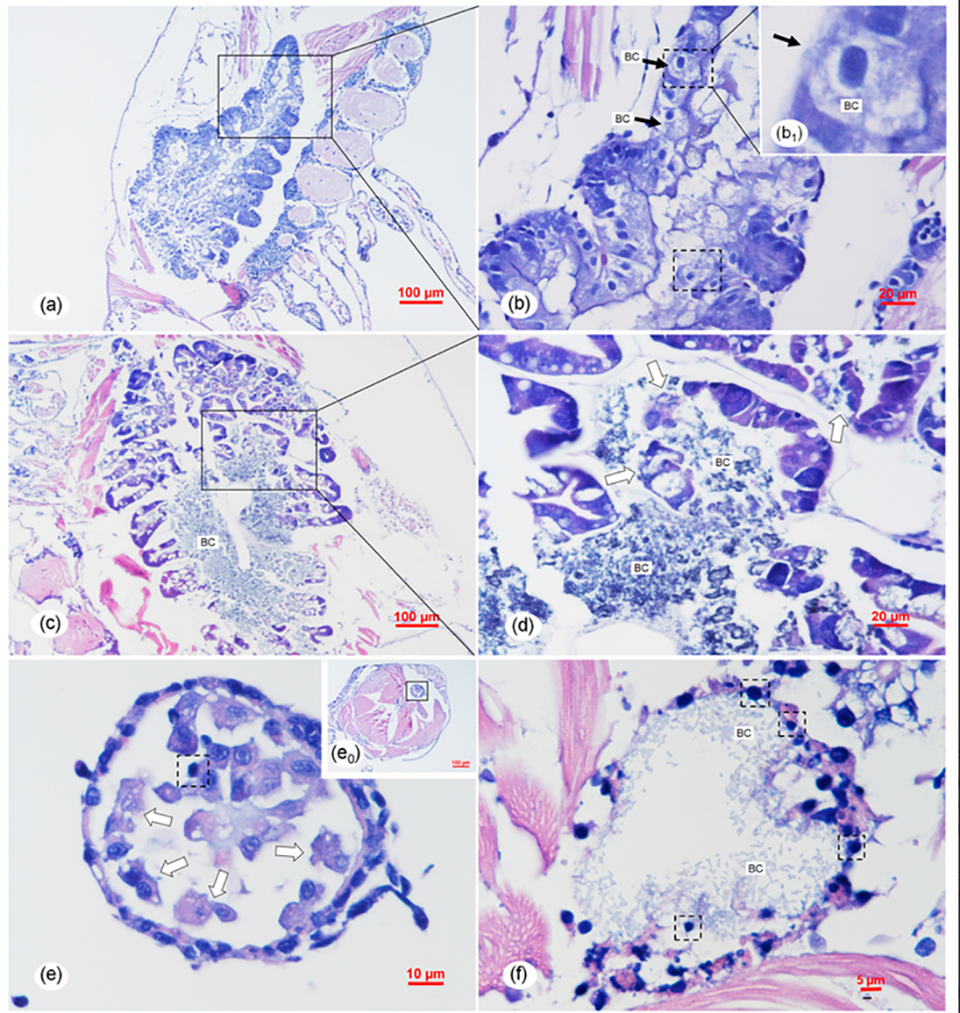
Based on our results, the gross histopathological [determined by microscopic examination] signs of TPD were not identical to those of AHPND and were also different from those caused by atypical V. parahaemolyticus. The unique gross histopathological signs of TPD suggested that the pathogenic mechanism of Vp-JS20200428004-2 were different from those of both typical VAHPND and atypical V. parahaemolyticus. For further exploration of the pathogenic mechanism of Vp-JS20200428004-2, more systematic comparative studies on the Vp-JS20200428004-2, typical VAHPND, and atypical V. parahaemolyticus should be designed and conducted; details about the functional genes, plasmids, and genome of Vp-JS20200428004-2 should especially be further investigated in the future.
To date, vibriosis, caused by various Vibrio spp., has been reported to occur at different developmental stages of L. vannamei, including nauplii (2 days), zoea (4 to 5 days), mysis (3 to 5 days), postlarvae (10 to 15 days) and juveniles. Vibrio alginolyticus was associated with the zoea 2 syndrome and the mysis mold syndrome in the stage of larvae, while V. alginolyticus and V. harveyi were associated with the bolitas syndrome.
The latter refers to a larvae syndrome in L. vannamei involving the detachment of epithelial cells from the intestine and hepatopancreas, which appeared as small spheres within the digestive tract. Apparently, the histopathological syndromes of the intestine and hepatopancreas of TPD caused by the Vp-JS20200428004-2 were different from that of bolitas syndrome. In addition, the clinical signs of TPD were distinct from the clinical signs of bolitas syndrome, which included reduced feeding, retarded development, sluggish swimming, and reduced escape mechanism. Thus, based on our results, we determine TPD to be a novel vibrio disease occurring in the PL stage of L. vannamei.
Perspectives
Based on our results of the systematic analysis including pathogenic agent isolation, identification and assays following the four criteria of Koch’s postulates [four criteria designed to establish a causative relationship between a microbe and a disease], we confirmed that a novel V. parahaemolyticus (Vp-JS20200428004-2) was the causative agent associated with the emerging TPD that affected shrimp farming in China in 2020.
The new pathogen shows high virulence to shrimp PLs and can cause acute and severe histopathological changes in the hepatopancreas and midgut. The risk of epidemic disease and PL losses caused by this new pathogen deserves further attention.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-
Qingli Zhang, Ph.D.
Corresponding author
Yellow Sea Fisheries Research Institute
Chinese Academy of Fishery Sciences
Key Laboratory of Marine Aquaculture Disease Control
Ministry of Agriculture
Key Laboratory of Marine Aquaculture Epidemiology and Biosecurity
Qingdao 266071, China
Editor’s note: This article has 12 co-authors, but only the corresponding author is included.
Tagged With
Related Posts

Health & Welfare
Culture of Pacific white shrimp juveniles in super-intensive conditions
Results of this study showed that L. vannamei juveniles can be cultured at high densities with adequate survival, growth and feeding efficiency, and with minimal water exchange or full water reuse through recirculation.

Health & Welfare
Influence of stressors on shrimp susceptibility to White Spot Disease, Part 2
Study examines the impact of environmental stressors like dissolved oxygen, nitrogenous compounds and pH on shrimp contracting White Spot Disease.

Health & Welfare
AHPND is a chronic disease in Pacific white shrimp from Latin America
There is a new phase of infection for Acute Hepatopancreatic Necrosis Disease on Latin American shrimp farms, a contrast to observations in Southeast Asia.

Intelligence
10 takeaways from GOAL 2019 in Chennai, India
The Global Aquaculture Alliance held its GOAL conference in Chennai, India, and recruited a host of experts in various fields to share their expertise.


