Only one viral disease, lymphocystis, has been reported
Yellow perch (Perca flavescens) is an emerging aquaculture candidate in the United States whose diseases are currently being elucidated. Like all cultured fish, yellow perch are susceptible to a wide range of disease syndromes, including those with infectious and noninfections causes. Current intensive aquaculture practices, like the use of high stocking densities and maximal feeding rates, often result in excessive stress to fish and compromised fish health.
Disease problems are one of the most common causes of fish loss in aquaculture, so it is important to recognize the early signs of disease and identify their causes. Only then can appropriate methods be used to remedy further escalation of problems in aquaculture facilities.
Parasites
Various external and internal parasites affect yellow perch. External protozoan parasites such as Trichodina sp., Epistylis sp., Ichthyophthirius multifiliis, and the multicellular monogeneans can have serious health consequences when fish are heavily infected.
External pests
Heavy infestations of the ciliated protozoan Trichodina sp. cause skin lesions and fin and tail erosion, as well as gill pathology. Epistylis sp., a stalked ciliated protozoan that feeds on material in the water column, attaches to the skin of the fish and causes small lesions at the site of attachment. This organism can also be found on the walls of tanks or along the edges of ponds with high organic loads.
Ichthyophthirius multifiliis, which causes “ich” or “white spot disease,” has been reported to cause significant mortalities in cultured yellow perch populations. This ciliated parasite burrows into the skin and gill tissue, directly affecting the health of the fish while protecting itself from the outside environment and chemotherapeutic treatments. Monogeneans can also attach to the skin and gills and cause significant irritation of the tissues.
Internal parasites
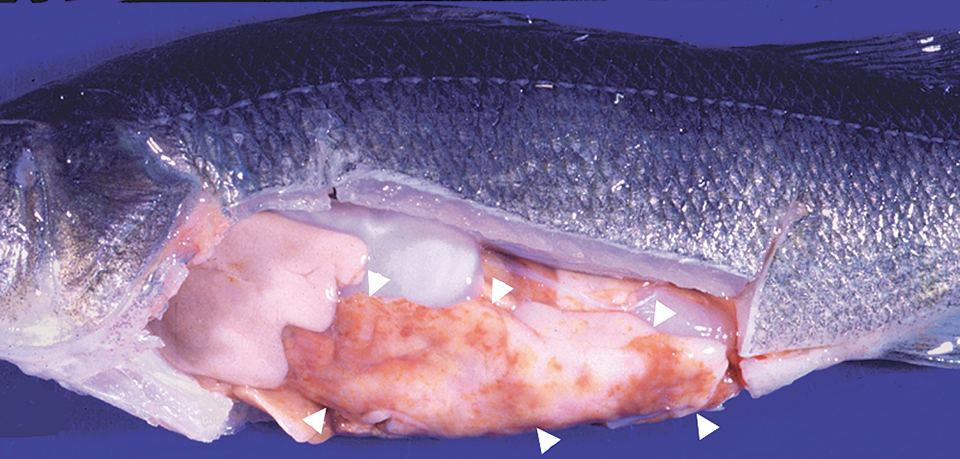
A number of other parasites infect the internal tissues of cultured yellow perch. Heterosporis sp. is a protozoan parasite that infects the flesh of yellow perch and makes it appear white in color, similar to that seen in fillets that are freezer-burned. The small protozoan parasite Myxobolus neurophilus has been occasionally observed in the brains of clinically diseased fish.
Digenetic trematodes, tapeworms, roundworms, and coccidia have also been reported in yellow perch. However, these parasites are generally only a problem in wild-caught or pond-raised yellow perch, since many of the parasitic life cycles cannot be completed in an enclosed aquaculture facility.
Bacterial diseases
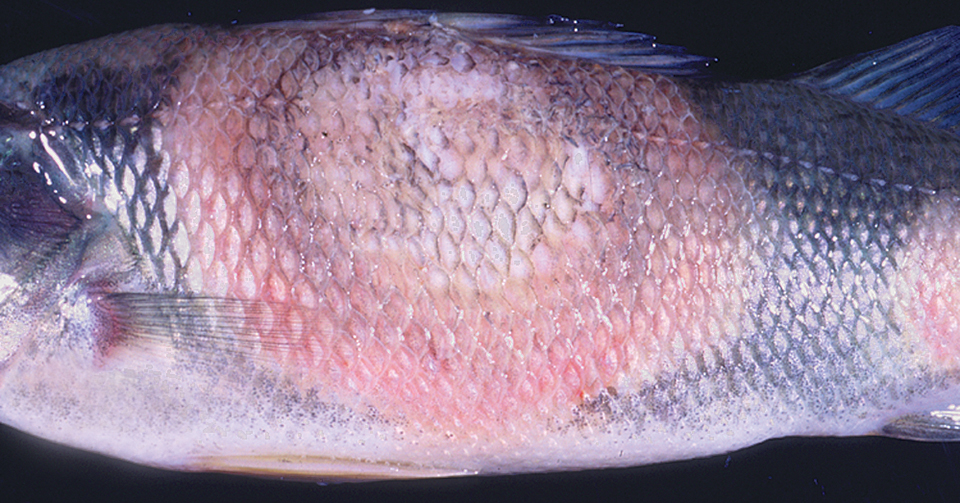
Pathogenic bacteria commonly reported in cultured yellow perch populations include Flavobacterium columnare, Aeromonas hydrophilia, and Mycobacterium sp. Flavobacterium columnare causes “columnaris disease” in many species of warmwater fish. One of the most serious pathogens of cultured yellow perch, it causes scale loss, hemorrhaging, tissue ulceration, and death. A different species of Flavobacterium affects the gill tissue of the fish, causing “bacterial gill disease” which hinders oxygen exchange. Aeromonas hydrophilia, a skin pathogen, often causes disease secondary to poor water quality or injury.
Mycobacterium sp. cause disease in numerous species of wild and cultured fish, including yellow perch. Infected yellow perch can develop a variety of signs that include anorexia, nonhealing skin lesions, and a chronic wasting syndrome. This disease is not treatable, and infected fish are unsuitable for human consumption. In addition, this is a zoonotic pathogen capable of causing disease in humans. When identified, all infected fish should be eliminated and the aquaculture systems disinfected.
Viral disease
Only one viral disease, lymphocystis, has been reported in cultured yellow perch. This iridovirus virus causes superficial skin and fin lesions in yellow perch, though infections are rarely fatal.
Fungal diseases
As in most species of fish, fungal diseases in yellow perch are generally secondary to poor water quality, poor management, or preexisting lesions on the skin, fins, or gills. They are most commonly caused by the genus Saprolegnia.
Noninfectious problems
Noninfectious problems that can result in high morbidity and mortality in a cultured yellow perch population include poor water quality and nutritional excesses or insufficiencies. Poor water quality conditions (i.e., high total ammonia-nitrogen, suboptimal temperature, gas supersaturation) can lead to increased stress, decreased immune responses, gill pathology, and secondary fungal, bacterial, and parasitic infections.
Diets high in lipid content are linked to hepatic lipidosis, commonly called “fatty liver syndrome,” and can lead to ill-thrift and chronic mortalities. Rancid diets can lead to steatitis or “brown fat disease” in the animals’ abdominal fat.
Disease prevention and control
Disease agents can enter and be spread in a fish production facility via the water, feed, fish, equipment and personnel. Thus, any new fish brought into a facility should be quarantined and appropriate biosecurity measures strictly enforced. Because many clinical signs of disease in fish are similar, a veterinarian or trained fish health professional should be consulted for an accurate diagnosis and appropriate corrective recommendations.
Treatment often is simply a matter of correcting inappropriate water quality parameters or management protocols. For specific diseases such as those caused by bacterial pathogens, culture and specific identification, as well as drug sensitivity testing are imperative for effective and lawful therapeutic treatment. However, if a specific pathogen is identified, a veterinarian must be consulted for any extralabel drug therapy, as no compounds are presently approved by the U.S. Food and Drug Administration for the treatment of any disease in yellow perch.
(Editor’s Note: This article was originally published in the August 2003 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Stephen A. Smith, DVM, Ph.D.
Aquatic Medicine Program
Department of Biomedical Sciences and Pathobiology
Virginia-Maryland Regional College of Veterinary Medicine
Virginia Polytechnic Institute and State University
Blacksburg, Virginia 24061-0442 USA[117,100,101,46,116,118,64,55,104,116,105,109,115,116,115]
-
Kathleen H. Hartman, DVM, Ph.D.
Aquatic Medicine Program
Department of Biomedical Sciences and Pathobiology
Virginia-Maryland Regional College of Veterinary Medicine
Virginia Polytechnic Institute and State University
Blacksburg, Virginia 24061-0442 USA -
David J. Pasnik, DVM, M.S.
Aquatic Medicine Program
Department of Biomedical Sciences and Pathobiology
Virginia-Maryland Regional College of Veterinary Medicine
Virginia Polytechnic Institute and State University
Blacksburg, Virginia 24061-0442 USA
Related Posts

Innovation & Investment
AquaBounty, with new RAS facility, hopes to win public support for GM salmon
Ron Stotish, CEO of AquaBounty Technologies, believes genetically modified salmon is no threat to its opponents and the outlook for AquAdvantage is good. With its purchase of the Bell Fish Co. RAS facility, commercialization will soon commence.

Aquafeeds
Buggin’ out: Tapping the potential of insect meal in aquaculture
Black soldier flies are gaining interest as a leading alternative ingredient in aquafeeds. But will the “ick” factor be a turn-off? Advocate contributor Clare Leschin-Hoar investigates.

Health & Welfare
Crowder/grader units improve harvest efficiency in large circular tanks
The use of larger and deeper tanks can reduce building, labor and other aquaculture production costs. However, the ability to grade and transfer large numbers of fish is more challenging when using large tanks. In a comparison of the effectiveness of a purse seine and a hinged clamshell to crowd fish in large tanks, the latter was easier to control and less stressful to salmonids. With a slotted bar rack in a side panel of the clamshell crowder, fish were simultaneously graded in size.

Health & Welfare
Live feed enrichment with probiotics
Probiotics can provide needed micronutrients that prime immune responses in larval fish, thus increasing their survival in culture. Probiotic dosing can be applied via immersion, microcosm approaches and enrichment of live and formulated feeds.


