Halophytic bacteria growth inhibited when exposed to low-salinity water

Low-salinity shrimp culture in southern Ecuador is done at inland farms using underground water that is pumped into 0.5- to 1.0-hectare (ha) ponds with liners and plastic greenhouse covers. Paddlewheel aeration is continuous during the whole production cycle, which can yield 7-10 metric tons (MT) per ha in 90 to 120 days.
The hatcheries that supply postlarvae to these farms acclimate the shrimp in water from 30 to 5 ppt salinity before transportation to the farms (Table 1). Once at the farms, postlarvae are further acclimated to water with 2 ppt salinity in pondside tanks for direct stocking or in nursery ponds before they are finally stocked in grow-out ponds.
Ching, Acclimation protocol, Table 1
| Salinity Range | Acclimation Time |
|---|
Salinity Range | Acclimation Time |
|---|---|
| 20-30 ppt | 2 ppt reduction every 20 minutes |
| 15-20 ppt | 2 ppt reduction every 30 minutes |
| 10-15 ppt | 1 ppt reduction every 30 minutes |
| 5-10 ppt | 1 ppt reduction every hour |
Monitoring, results
In research by the authors, the first set of bacteriological analyses consisted of three samples taken from two hatchery tanks containing water at 30 ppt salinity that provided postlarvae to each farm. Macerates of P.L.6 were cultured in agar, and Vibrio counts in colony-forming units per gram (CFU/g) were recorded either as yellow (sucrose-positive) or green (sucrose-negative) colonies.
A second set of these analyses were performed when P.L.12 postlarvae arrived at each farm in 5-ppt salinity water before they were stocked. The last set of analyses was done either in the nursery pond or in the grow-out pond during the first days of culture. Green colonies of Vibrio were later identified as V. parahaemolyticus.
Postlarvae from the hatchery tank with direct stocking averaged 442,400 yellow CFU/g and 29,933 green CFU/g (Fig. 1), while the postlarvae with a nursery phase had 390,000 yellow CFU/g and 20,933 green CFU/g (Fig. 2).
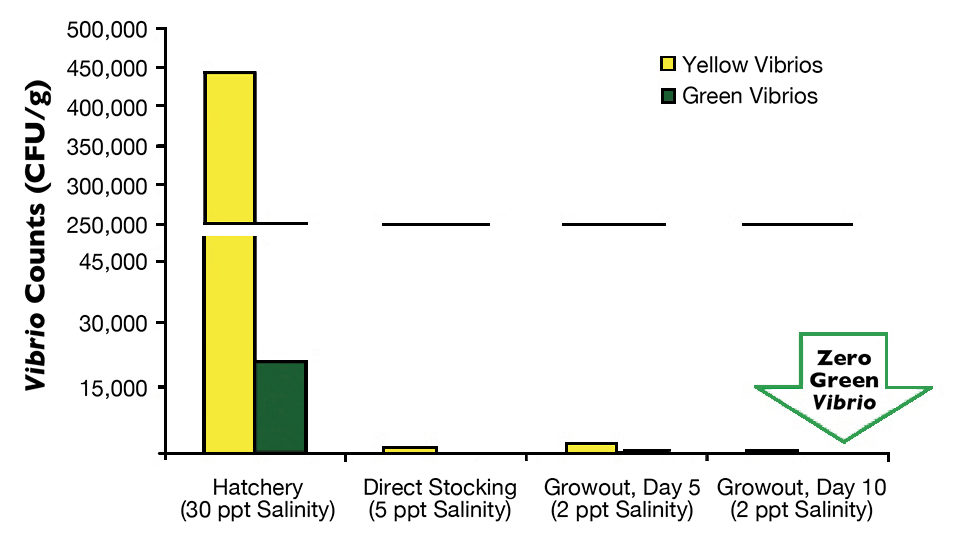
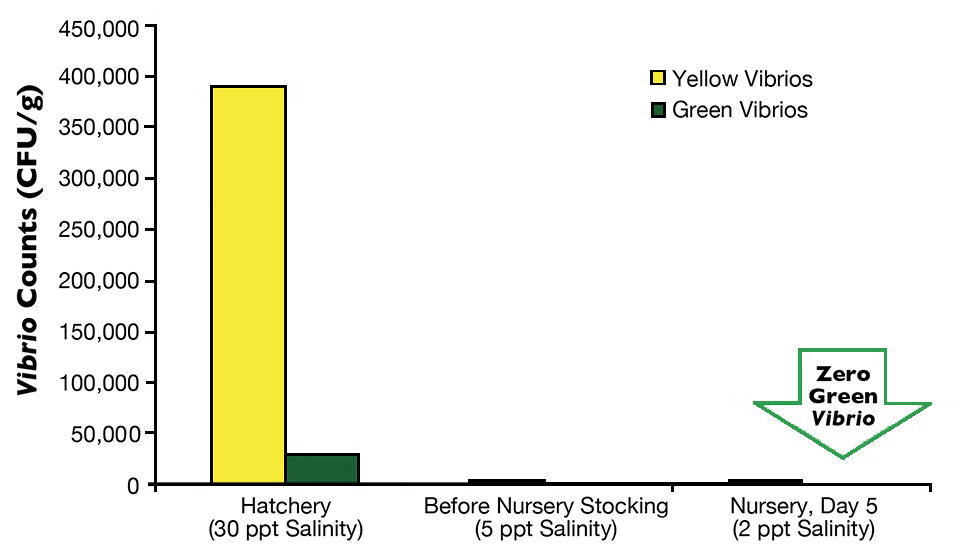
On arrival at the farms, an average of three samples of P.L.12 taken randomly from three transportation bags with 5-ppt salinity water indicated that in the farm with direct stocking, yellow Vibrio counts went down to 1,236 CFU/g, and green Vibrio counts went down to less than 100 CFU/g. At the other farm, average counts of yellow colonies went down to 3,000 CFU/g, and green colonies fell to 102 CFU/g.
Early culture
Finally, the last monitoring of Vibrio species in postlarvae was carried out during the first days of culture, either in the nursery pond for the two-phase farm or in a growout pond for the direct-stocking farm. In both cases, green colonies of V. parahaemolyticus were eradicated from the postlarvae in five days in the nursery pond and in 10 days in the growout pond. The difference between results for these two farms may be attributed to the higher minimum oxygen levels in the nursery pond (5.0 mg/L) than in the growout pond (4.0 mg/L).
Monitoring of the farms continued during the growout period to a final salinity of 3 ppt at harvest. It was noticed that V. parahaemolyticus never appeared again in the samples taken from the hemolymph and hepatopancreas tissues of shrimp, or even from water samples.

Perspectives
Vibrios are known as halophytic bacteria, meaning they grow well in high-salinity aquatic environments, and their growth is inhibited when they are exposed to low-salinity water. However, when shrimp postlarvae are infected with pathogenic Vibrio bacteria at very high concentrations in the hatchery, disease can become uncontrollable.
There is a good chance that the infection levels determine the fate of these postlarvae at farms, as is the case with early mortality syndrome (EMS) caused by a pathogenic strain of Vibrio parahaemolyticus. Even if infected postlarvae are cultured in freshwater, mortalities may occur during the first days of culture.
(Editor’s Note: This article was originally published in the November/December 2014 print edition of the Global Aquaculture Advocate.)
Authors
-
Dr. Carlos A. Ching
Aquaculture Manager
Nicovita – Vitapro S.A.
Av. Argentina 4793
Callao, Lima, Peru -
Ing. Juan Portal
Technical Assistance Manager
Nicovita – Vitapro S.A. -
Ing. Alfredo Salinas
Production Manager
Orocam
Huaquillas, Ecuador
Tagged With
Related Posts

Health & Welfare
Acclimating shrimp postlarvae before pond stocking
Shrimp postlarvae acclimation before stocking into the various growout systems (ponds, raceways, tanks) is a critical – and often overlooked, sometimes taken for granted – step in the shrimp culture process. Various water quality parameters should be changed slowly so that the young shrimp have the time to gradually adapt to the new conditions.

Aquafeeds
Biofloc consumption by Pacific white shrimp postlarvae
The stable isotopes technique with δ13C and δ15N can be used to determine the relevance of different food sources to shrimp feeding during the pre-nursery phase of Litopenaeus vannamei culture. During this trial, different types of commercial feed, microalgae, Artemia sp. nauplii and bioflocs were used as food sources.

Intelligence
As ocean temperatures rise, so too will vibrio outbreaks
A study using a half-century of data has linked climate change and warming sea temperatures with an increase in illnesses from the common vibrio bacteria. Shellfish growers, fighting a particularly virulent strain of Vibrio parahaemolyticus, are changing their harvest protocols.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.


