Addition of phytoplankton and microscopic ‘wheel animals’ can improve larval performance

In shrimp farming systems, the microbial community plays an important role in recycling nutrients, decreasing the anoxic zones in ponds and alleviating the nutrient load in wastewater, while providing an additional nutrition source for the cultured shrimp in semi-intensive and intensive systems.
The use of plankton in intensive shrimp farming systems yields better performance of the cultured organisms, as it improves the content of essential amino acids and highly unsaturated fatty acids in the tissues of cultured shrimp.
Study setup
This study was carried out at the Sustainable Mariculture Laboratory of the Fisheries and Aquaculture Department of the Rural Federal University, Pernambuco, Recife, Brazil, to assess shrimp performance in a biofloc system with the addition of diatoms (Navicula sp.) and rotifers (Brachionus plicatilis).
For the duration of the experiment, the light intensity was kept at about 1,000 lux using a fluorescent lamp with a natural photoperiod. No water exchange was done during the experimental period, except for the addition of dechlorinated freshwater to compensate for evaporation losses.
Molasses was added once a day as a carbon source to maintain the carbon:nitrogen ratio at 12:1. Hydrated lime was used to maintain alkalinity and pH above 100 mg/L and 7.5, respectively. The experimental shrimp were fed daily with a commercial shrimp feed (40 percent crude protein), and rations were adjusted daily based on the estimated shrimp consumption, mortality rate and unconsumed feed.
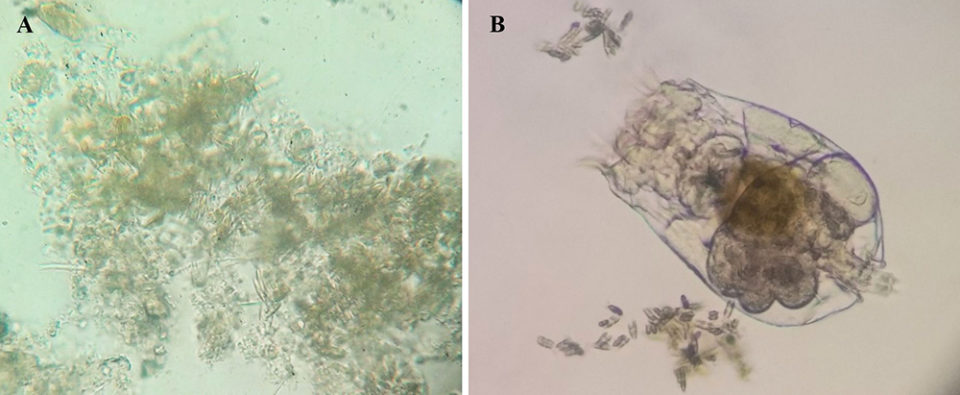
First test – diatom addition
We evaluated the performance of Pacific white shrimp (Litopenaeus vannamei) postlarvae in a biofloc system during 20 days with the addition of the diatom Navicula sp. The shrimp (17.7 ± 0.02 mg,) were stocked at 2,500 shrimp/m3 in the experimental units. Diatoms were added at a concentration of 5 x 104/mL to the culture tanks on days 1, 5, 10 and 15.
Five days prior to stocking the shrimp, water from a matrix tank (0.12 mg/L total ammonia nitrogen, 2.2 mg/L nitrite nitrogen, alkalinity of 100 mg calcium carbonate/L, and 27 mL/L settleable solids) was mixed and used to fill 12 black-plastic tanks (usable dimensions of 50 x 35 x 23 cm, equivalent to a volume of 40 L) to approximately 50 percent of their volume, and the remaining was filled with seawater.
Second test – diatom and rotifer additions
We also evaluated the performance of Pacific white shrimp postlarvae during 35 days in a biofloc system with the addition of the diatom Navicula sp. and rotifers (B. plicatilis). The shrimp (16.2 ± 0.03 mg) were stocked at 2,500 shrimp/m3 in the experimental units. Diatoms (at a concentration of 5×104/mL) and rotifers (at a concentration of 30 rotifers/mL) were added to the culture tanks on days 1, 5, 10, 15, 20, 25 and 30.
Five days prior to stocking the shrimp, water from a matrix tank (0.2 mg/L total ammonia nitrogen, 0.5 mg/L nitrite nitrogen, 2.2 mg/L nitrate nitrogen, alkalinity of 134.7 mg calcium carbonate/L, and total suspended solids of 206 mg/L) was mixed and equally distributed to fill 12 black-plastic tanks (useful dimensions of 50 x 35 x 23 cm, equivalent to 40 L).
Results
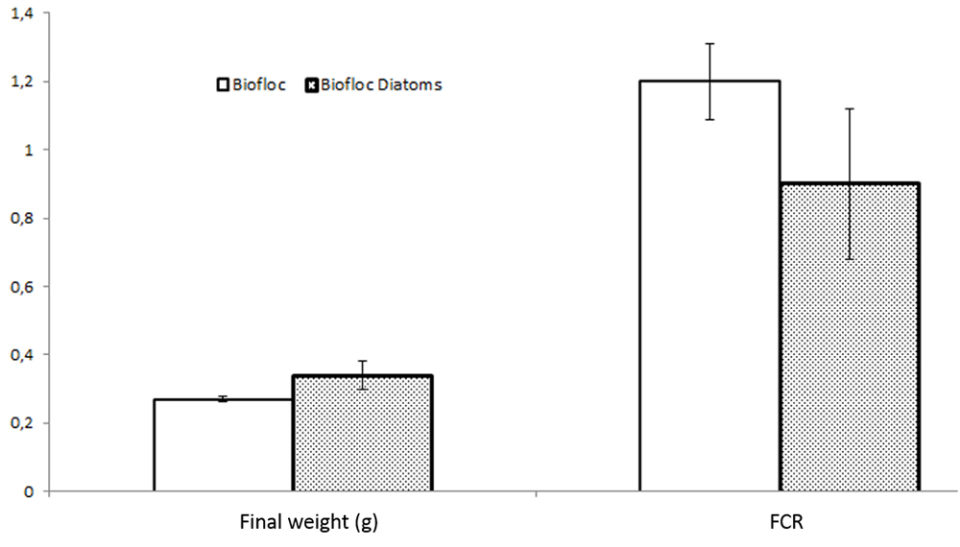
In the first test, the shrimp survival rates were all above 87 percent during the 20-day experimental period. The feed conversion rate (FCR) of 0.9 and the final weight of 0.34 grams of shrimp in the tanks with added diatoms were significantly better (P < 0.05) than for shrimp in tanks without added diatoms (1.2 FCR; 0.27g final weight).
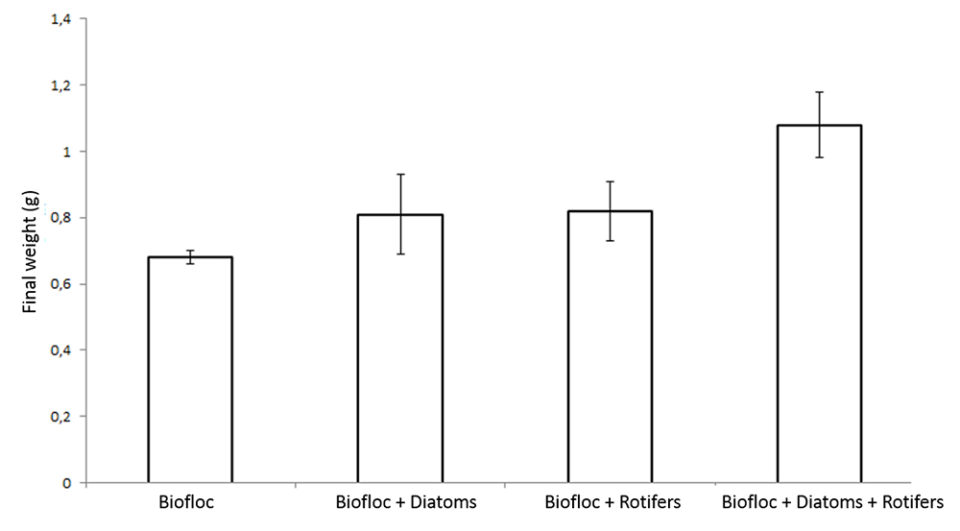
In the second test, shrimp survival rates were all above 70 percent in the tanks without added diatoms and rotifers, and 85 to 91 percent in the tanks with added diatoms and rotifers. The final weight of shrimp in the tanks with added diatoms and rotifers ranged from 0.81 to 1.08 grams, and 0.68 grams in the tanks without added diatoms and rotifers. Shrimp receiving diatoms and rotifers had an FCR ranging from 0.92 to 1.37, and shrimp in tanks without added diatoms and rotifers had an FCR of 1.94 tanks.
The positive effect of diatom and rotifer additions on the performance parameters of shrimp indicate that Navicula sp. and B. plicatilis serve as natural food sources for postlarval L. vannamei in biofloc systems. They probably provide important nutrients like essential amino acids and highly unsaturated fatty acids, essential to shrimp survival and growth.
Perspectives
The results of our study indicate that the addition of diatoms (Navicula sp.) and rotifers (B. plicatilis) to larval shrimp tanks every five days provides a significant natural food source for shrimp in their early stages in biofloc systems.
References available from the corresponding authors.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-

Dr. Luis Otavio Brito da Silva
Corresponding Author
Professor Adjunto A
Departamento de Pesca e Aquicultura
Universidade Federal Rural de Pernambuco
Recife, Brazil -

Jéssika Lima de Abreu
Departamento de Pesca e Aquicultura
Universidade Federal Rural de Pernambuco
Recife, Brazil -

Clarissa Vilela Figueiredo da Silva Campos
Departamento de Pesca e Aquicultura
Universidade Federal Rural de Pernambuco
Recife, Brazil -

Yllana Ferreira Marinho
Centro de Ciências Humanas, Naturais, Saúde e Tecnologia
Universidade Federal do Maranhão
Pinheiro, Maranhão, Brazil -

Marcele Trajano de Araújo
Departamento de Pesca e Aquicultura
Universidade Federal Rural de Pernambuco
Recife, Brazil -

Dr. Alfredo Olivera Gálvez
Corresponding Author
Departamento de Pesca e Aquicultura
Universidade Federal Rural de Pernambuco
Recife, Brazil
Tagged With
Related Posts

Aquafeeds
Novel reactor developed for indoor, high-density production of diatoms
The development of this reactor for the indoor cultivation of non-suspended microalgae like important diatoms such as Amphora spp., and the cellular dry matter values produced in this study will help bio-filming science support the development and improvement of in situ feed supplementation for fish and shrimp ponds, particularly in desert environments.

Aquafeeds
Optimizing culture of the weissflogii diatom
The diatom Thalassiosira weissflogii is important in the aquaculture industry to feed shrimp and shellfish larval stages in hatcheries. This study examined culture conditions for this diatom and determined that it can be successfully cultured semi-continuously and without population crashes.

Aquafeeds
Biofloc consumption by Pacific white shrimp postlarvae
The stable isotopes technique with δ13C and δ15N can be used to determine the relevance of different food sources to shrimp feeding during the pre-nursery phase of Litopenaeus vannamei culture. During this trial, different types of commercial feed, microalgae, Artemia sp. nauplii and bioflocs were used as food sources.

Health & Welfare
Aquamimicry: A revolutionary concept for shrimp farming
Aquamimicry simulates natural, estuarine production conditions by creating zooplankton blooms as supplemental nutrition to the cultured shrimp, and beneficial bacteria to maintain water quality. Better-quality shrimp can be produced at lower cost and in a more sustainable manner.

