Results show a risk factor for AHPND in Pacific white shrimp

Acute hepatopancreatic necrosis disease (AHPND), caused by specific strains of the bacterium Vibrio parahaemolyticus (VpAHPND), has been reported in shrimp cultured in Southeast Asia and the Americas since 2009. The clinical signs of the disease include atrophied hepatopancreas (HP), an empty gastrointestinal tract, a milky appearance of the stomach and lethargy. This bacterial disease has caused up to 100 percent mortality in populations of farmed Pacific white shrimp (Penaeus vannamei) and black tiger shrimp (P. monodon), and collective economic losses due to AHPND and other shrimp diseases are estimated at $23.6 billion globally with a further loss of $7 billion in feed sales.
In some cases, VpAHPND has been reported in co-infection with the spore-forming microsporidian Enterocytozoon hepatopenaei (EHP). Although EHP does not appear to cause mortality, EHP-infected animals would be more vulnerable to AHPND, which may cause substantial mortality in shrimp ponds. And EHP-infected shrimp have an increased susceptibility to VpAHPND, suggesting that shrimp weakened by EHP would succumb to secondary pathogens, such as VpAHPND.
For several years, white spot syndrome virus (WSSV) has spread worldwide throughout much of Asia, the Americas, Europe, Africa, Middle East and Australia. In some cases, WSSV has been detected in healthy animals without clinical symptoms or mortalities, indicating that infection by WSSV does not necessarily result in high mortality, and secondary infection by opportunistic pathogens, such as Vibrio spp., would expedite the onset of the disease.
In 2017, P. vannamei mortality rates of up to 60 percent were reported in the Philippines. The shrimp exhibited clinical signs of AHPND but were confirmed by PCR to be positive for both VpAHPND and WSSV. From this case, we hypothesized that WSSV infection without clinical symptoms would allow VpAHPND to cause faster and higher mortality of pond cultured shrimp than those infected with VpAHPND alone.
This article – adapted and summarized from the original – reports on a study designed to support this hypothesis by simulating the co-infection with WSSV and VpAHPND in juvenile shrimp P. vannamei under laboratory conditions. As a result, we demonstrated that shrimp initially exposed to WSSV were easily infected by VpAHPND, but hardly recovered from the disease according to histopathology examination. In addition, using qPCR assay and immunohistochemistry [IHC; the most common application of immunostaining, involving the process of selectively identifying antigens (proteins) in cells of a tissue section using the binding of antibodies specifically to antigens in biological tissues] examination, we determined that WSSV infection could be accelerated by the secondary VpAHPND infection. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant number NRF-2018R1C1B5086350).
Study setup
Postlarval P. vannamei were sourced from a local shrimp farm (Jeju Province, South Korea) and transported to the Institute of Marine Sciences of Jeju National University in South Korea. Shrimp were reared to an average weight of 0.5 ± 0.05 grams in 96-liter maintenance tanks with aerated artificial seawater at 25 to 28 degrees-C and 30 ppt salinity. For the co-infection experiment, juvenile shrimp (average 0.5 grams, N=80) were randomly selected from the maintenance tanks and distributed into experimental tanks. Before the co-infection experiment, representative shrimp were randomly selected and confirmed to be negative for both WSSV and VpAHPND by PCR.
For WSSV stocks, moribund P. vannamei with white spots on their cuticles were collected from an anonymous shrimp farm in Chungcheong Province located on the western coast of Korea. For VpAHPND bacterial stocks, the strain 13–028/A3 was previously isolated from the stomach of moribund P. vannamei from a farm in Vietnam and displaying whitish hepatopancreases (HP).
For detailed information on the experimental designs for WSSV and VpAHPND co-infection; qPCR assays; IHC examination of the gill tissues; histopathology examination of the HP tissues; and statistical analyses, please refer to the original publication.
Results and discussion
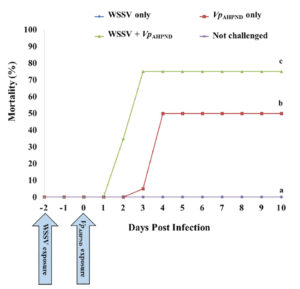
We studied co-infection with WSSV and VpAHPND in juvenile P. vannamei shrimp under laboratory condition, and the mortality rates were compared with single infection groups.
Our results showed that co-infection accelerated shrimp mortality compared with single infection (Fig. 1). We observed 0 percent mortality Group 1 (WSSV only), and cumulative mortality was 50 percent at day 4 post-infection (p.i.) in Group 2 (VpAHPND only). However, the mortality rate increased significantly in Group 3 (WSSV+VpAHPND) compared with the other groups (p < .05). In Group 3, the shrimp began to show mortality at day 2 p.i., and cumulative mortality reached 75 percent at day 3 p.i. During the 10-day experimental period, no mortality was observed in the negative control (Group 4, not challenged).
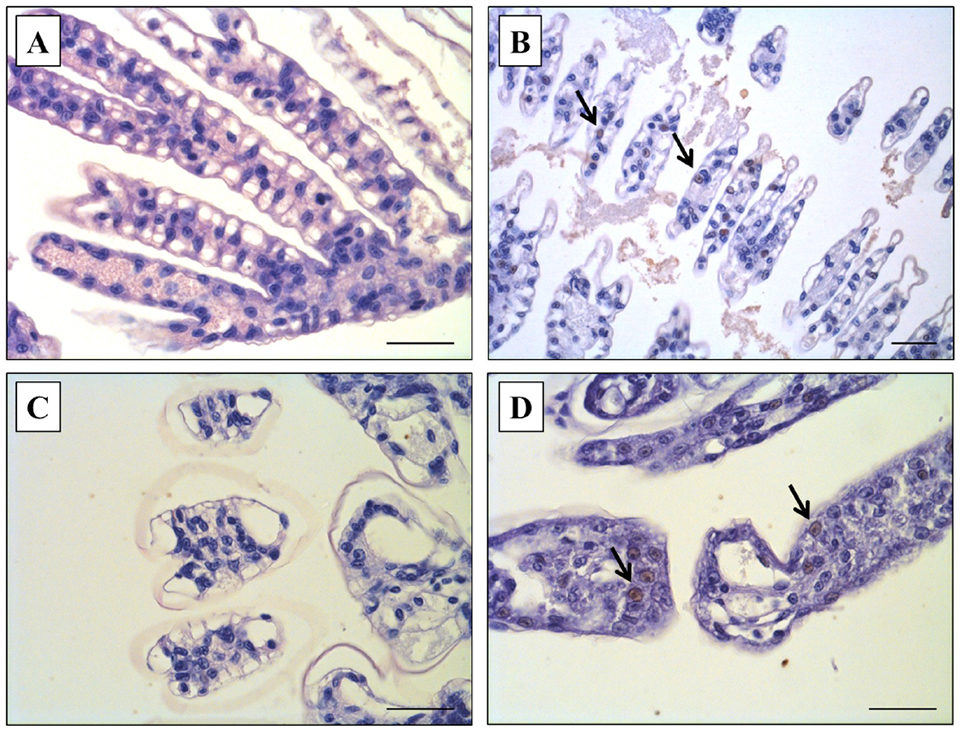
Our results also showed that co-infection increased the level of WSSV infection, which was confirmed via qPCR and IHC, and the levels of WSSV infection were markedly different between Group 1 (WSSV only) and Group 3 (WSSV + VpAHPND) on the termination day (Day 10). Based on qPCR analysis, WSSV was detected in shrimp in Group 3 (WSSV+VpAHPND) but WSSV was negative in shrimp exposed to WSSV only (Group 1). Also, by IHC examination, brown coloration against pink cytoplasm and purple nuclei, indicative WSSV-positive reactions were observed in the shrimp of Group 3 (WSSV+VpAHPND) (Fig. 2D) similar to the WSSV-positive sample previously prepared in the preliminary study (Fig. 2B). However, these WSSV-positive reactions were not seen in the shrimp exposed to WSSV alone (Group 1; Fig. 2A) and the negative control (Group 4, not challenged; Fig. 2C).
Co-infection decreased the recovery of VpAHPND infection. All experimental shrimp showed typical clinical symptoms of AHPND, including pale HP and empty gut, within three to four days after infection in Group 2 (VpAHPND only) and Group 3 (WSSV+VpAHPND), and VpAHPND infection was confirmed by qPCR and histopathology examination. From the qPCR analysis, VpAHPND was detected in dead shrimp collected from both Group 2 (VpAHPND only) and Group 3 (WSSV+VpAHPND) on Day 3 and 4.
By histopathology examination, representative shrimp from both Group 2 (VpAHPND only) and Group 3 (WSSV + VpAHPND) showed a typical terminal phase, characterized by massive bacterial infection and sloughing of hepatopancreatic tubule epithelial cells at the level of G3-4 (grade 4 is the most severe) on Day 3.
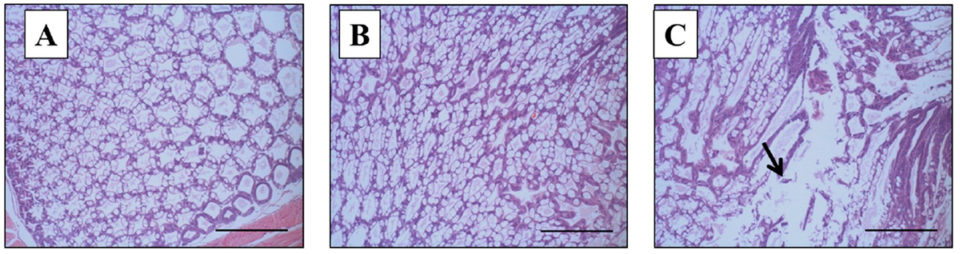
However, on the termination day (day 10), a remarkable difference in the histopathology of the HP of surviving shrimp was seen between group 2 (VpAHPND only) and Group 3 (WSSV+VpAHPND). Histopathology signs of the group 2 (VpAHPND only) survivors had an HP of normal appearance, and normal functions of B and R cells (Fig. 3B), the same as the negative control (Group 4, Fig. 3A). To the contrary, Group 3 (WSSV+VpAHPND) survivors showed typical AHPND histopathology signs in the HP, with massive bacterial infection and sloughing of hepatopancreatic tubule epithelial cells of G4 severity (Fig. 3C).
Infections with multiple pathogens (co-infection) are common in practical shrimp cultures. Several cases of shrimp vibriosis with WSSV infection have been reported with various penaeid species in different countries. There has been no prior detailed analysis on the roles of these pathogens in shrimp co-infected with WSSV and Vibrio. In shrimp farms, the WSSV infection without symptoms or mortality probably causes shrimp weakening and might trigger the VpAHPND infection.
We also observed declines in antioxidant and antiperoxidative enzymes in shrimp infected with VpAHPND, similar to WSSV in our ongoing study, and we suspect that VpAHPND infection may also have compromised the animals, increasing their susceptibility to other pathogens in ponds.
Intramuscular (IM) routes have been widely used for experimental infection of shrimp with WSSV. However, IM injection has not been considered as an infection route of VpAHPND. In addition, per os feeding can produce both WSSV and VpAHPND infection, but this is not a practical method for the dual-infection experiments because experimental shrimp were supposed to be infected twice within short intervals. Therefore, for the current bioassay, we selected the immersion (waterborne) method to co-infect shrimp with WSSV and VpAHPND. The synergistic effects influenced by the same infection routes have already been confirmed between WSSV and Vibrio spp.
Perspectives
To the best of our knowledge, our study is the first to examine the co-infection of WSSV and VpAHPND under laboratory conditions. In ponds, shrimp that were infected with WSSV (or other pathogens) did not always show clinical signs or mortalities. In those cases, even WSSV-positive shrimp without any clinical symptoms may cause higher and faster mortality than their disease-free counterparts, and the shrimp would hardly recover from the disease following a secondary infection, such as VpAHPND.
Also, the secondary infection would provoke WSSV infection or virus replication, as confirmed in the present study. Therefore, shrimp farmers should pay attention to the management of multiple infections (WSSV or other pathogens) in their ponds to prevent further losses in shrimp production.
References available in the original publication.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-

Jee Eun Han, DVM, Ph.D.
Laboratory of Aquatic Biomedicine
College of Veterinary Medicine
Kyungpook National University
Daegu 41566, Korea -
Ji-Eun Kim, MS
Institute of Integrated Technology
CJ CheilJedang, CJ Blossom Park
Suwon 16495, Republic of Korea -
Hayun Jo, MS
Institute of Integrated Technology
CJ CheilJedang, CJ Blossom Park
Suwon 16495, Republic of Korea -
Jong-Su Eun, Ph.D.
Institute of Integrated Technology
CJ CheilJedang, CJ Blossom Park
Suwon 16495, Republic of Korea -
Chorong Lee, Ph.D.
Department of Marine Life Sciences
Jeju National University
Jeju 63243, Republic of Korea -
Ji Hyung Kim, Ph.D.
Infectious Disease Research Center
Korea Research Institute of Bioscience and Biotechnology
Daejeon 34141, Republic of Korea -
Kyeong-Jun Lee, Ph.D.
Department of Marine Life Sciences
Jeju National University
Jeju 63243, Republic of Korea -
Jae-Won Kim, Ph.D.
Institute of Integrated Technology
CJ CheilJedang, CJ Blossom Park
Suwon 16495, Republic of Korea
Tagged With
Related Posts

Health & Welfare
EHP a risk factor for other shrimp diseases
Laboratory challenges and a case-control study were used to determine the effects of EHP infection on two Vibrio diseases: acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN).

Innovation & Investment
Aquaculture America 2017: Communication key to the future
This year’s Aquaculture America in San Antonio, Texas, provided significant learning and networking opportunities. It successfully brought together 14 U.S. aquaculture organizations and more than 1,600 participants from Europe, Asia, Africa and Australia.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
Clinical case report: EMS/AHPND outbreak in Latin America
Behind the successful control of Early Mortality Syndrome (EMS), or Acute Hepatopancreatic Necrosis Disease (AHPND), in a Latin American shrimp nursery.


