Critical support for a major shrimp farming industry

Aquaculture production in India has substantially improved during the last five years, especially after the introduction of Pacific white shrimp (Litopenaeus vannamei). Farmed shrimp production has almost tripled, from 130,000 tons in 2009 during the pre-vannamei period to 350,000 tons during 2014-15. The availability of imported, specific pathogen free (SPF) broodstock and quality seedstock provided much needed impetus to India’s brackishwater aquaculture sector.
The aquatic quarantine facility (AQF) set up at Chennai with the collaborative efforts of all stakeholders – the Ministry of Agriculture and Farmers’ Welfare (MoA&FW), Coastal Aquaculture Authority (CAA), Marine Products Export Development Authority (MPEDA), Rajiv Gandhi Centre for Aquaculture (RGCA), Indian Council of Agricultural Research (ICAR)-CIBA and the industry – has been playing an important role since the year of introduction in 2009.
Production intensification and emerging disease issues
However, progressive intensification of shrimp farming in India has exacerbated the epizootics and disease issues are affecting production. The work carried out at CIBA under the National Surveillance Programme for Aquatic Animal Diseases (NSPAAD) in India has thrown light on the emergence of the disease, shrimp hepatopancreatic microsporidosis, caused by Enterocytozoon hepatopenaei (EHP), in Indian shrimp farms.
There, this parasite has been implicated in stunted growth in farmed shrimp and related productions loses (Rajendran et al. 2016. Emergence of EHP in farmed Penaeus (Litopenaeus) vannamei in India. Aquaculture 454: 272–280).
Further, the study also revealed that white spot disease (WSD) caused by white spot syndrome virus (WSSV) continues to be the major killer of farmed shrimp, especially in the major shrimp producing states, such as Andhra Pradesh. Another interesting observation is the increasing incidence of infectious hypodermal and hematopoietic necrosis (IHHN) in farmed shrimp. Emerging of syndromes such as chronic mortality syndrome (CMS, popularly called as running mortality syndrome, or RMS by shrimp farmers), white faeces syndrome (WFS) and white muscle syndrome (WMS) have been also contributing to morbidity and mortality of farmed shrimp in India.
Considering the limited therapeutic options available for the control of diseases, only timely and early disease detection using sensitive and specific diagnostic tools, active disease surveillance, followed by proper health management measures would be the practical approach in containing the issues of existing and emerging diseases problems in brackishwater aquaculture.
Until 2008, most farmers in India tested black tiger shrimp (Penaeus monodon) seedstock for WSSV using polymerase chain reaction (PCR) in commercial laboratories prior to stocking ponds. This practice helped control the introduction of WSSV in the rearing systems and allowed for the successful management of black tiger shrimp crops.
However, after the introduction of the exotic, specific pathogen free (SPF) Pacific white shrimp in 2009 in India, farmers gave away the practice of PCR screening of shrimp seed, possibly due to their misconception that the seed originated from SPF stock and that PCR testing was not required.

On the contrary, the disease surveillance program revealed high prevalence of WSD, EHP, IHHNV and other syndromes. In this backdrop, CIBA – being a National referral laboratory for brackishwater aquatic animal diseases (NRLD) – realized the need of screening for WSSV before seedstock stocking, considering the increasing trend of WSSV-related crop failures.
This initiated a program for “capacity building and ring test/harmonization of PCR diagnosis of aquatic animal diseases” for the benefit of the shrimp aquaculture sector in the country, in association with the Coastal Aquaculture Authority (CAA) and Rajiv Gandhi Centre for Aquaculture (RGCA) under the Marine Products Export Development Authority (MPEDA), the central government agencies. The PCR diagnostic testing requires a significant skill set and validation to ensure the highest possible sensitivity and specificity, so that credible and quality PCR testing services could be made available to the farmers.
Ring tests, harmonization of PCR diagnosis
One of the purposes of ring tests, also known as inter-laboratory performance, is to assure the farmers or regulatory officials that the results provided are accurate and specific (Pantoja et al. 2011. Ring Tests Compare PCR Results from Diagnostic Laboratories. Global Aquaculture Advocate, May-June 2011: 36-37). This would help farmers make better selection of seedstock and take appropriate management decisions during the grow-out culture, based on the results of PCR testing services provided by the private PCR labs approved after the ring test.

The other purpose of PCR ring tests is to determine if the test methods in use are reliable and reproducible. Lack of uniform laboratory results and consensus among PCR laboratories necessitate greater attention to standardization and harmonization. The participating laboratories would play a major role in providing accurate diagnostic services to the aquafarmers in the country. This effort would contribute significantly to prevention of diseases and the sustainability of the aquaculture sector in the country.
The first-ever PCR ring test was conducted in India by ICAR-CIBA Chennai in 2005, under a regional project titled “Application of PCR for improved shrimp health management in the Asian region” implemented in India, Indonesia, Thailand and Australia, with key partners including ACIAR, CSIRO and AusVet Services in Australia; MPEDA, CIBA and the College of Fisheries, Mangalore in India; Mahidol University, BIOTEC and FAO-NACA in Thailand; the Ministry of Marine Affairs and Fisheries in Indonesia.
A total of 46 applications were received from private and government PCR-service-providing labs, hatcheries and research institutions. Out of these, 21 laboratories across the shrimp farming regions of Andhra Pradesh and Tamil Nadu successfully completed the PCR ring test. The second PCR ring testing exercise was conducted in the year 2009 by ICAR-CIBA and MPEDA, in which 34 laboratories participated and 31 participants provided their results – 21 laboratories successfully completed the second PCR ring testing exercise in the year 2009.
PCR training and the 2017 ring test
CIBA initiated the process of ring test involving the CAA, the regulatory body and RGCA. An Expression of Interest (EOI) for participation in the ring test was invited by CAA and widely publicized through advertisements in newspapers and on websites of CAA, CIBA and RGCA.
The exercise comprised three stages. In the first stage, PCR infrastructure available at various laboratories was established through inspections by a team from CIBA and RGCA. This was followed by a hands-on training program during 3-5 October 2016 for laboratory technicians at CIBA, including PCR training for three days, with theoretical and practical classes on nucleic acids, principles and practice of PCR for WSSV, EHP and an RNA virus, infectious myonecrosis virus (IMNV). A training manual was provided to the trainees, with comprehensive information on important shrimp diseases, basics of nucleic acids and PCR, protocols for shrimp sampling, nucleic acid extraction, performing PCR, electrophoresis and reporting the PCR results, in addition to a section on trouble-shooting.

The final phase of the “ring test” included sample distribution and evaluation of the results submitted by the participating laboratories. Each laboratory was provided with a panel of four coded tissue samples comprising positive and negative samples for WSSV, fixed in 95 percent ethanol for testing during the first week of January 2017. Participation in the ring test was voluntary and the laboratories were free to use the protocol of their choice. A standard report format was provided and the laboratories were given a maximum of five working days to analyses the samples and submit results. For the reports to be considered complete, the primer sets or commercial kits, extraction methods, PCR conditions and gel photographs were expected to be included. Laboratories which employed real-time PCR were expected to include the chromatograms in the results section of the report.
Results were kept confidential and only provided to the participants through the specific codes, known to only individual laboratories and the nodal institute, ICAR-CIBA and RGCA. Since the purpose of the ring test is not only to determine proficiency, but to help improve performance, feedback was also provided to those laboratories experiencing problems with the analysis, so that they can provide quality services to the farmers.
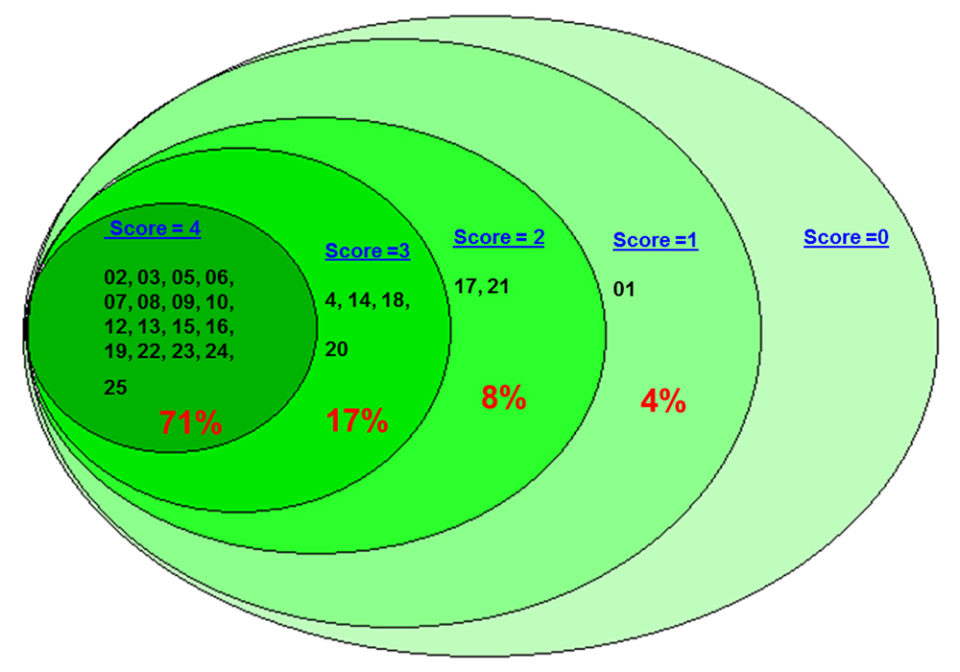
A total of 25 laboratories – including 8 labs from Tamil Nadu, 11 from Andhra Pradesh, three labs from Karnataka and three labs from Gujarat – participated in the ring test 2016-17. Twenty-four out the 25 laboratories performed the tests and submitted the results. A total of 71 percent (17/24) of the labs scored 4 and passed the ring test by correctly diagnosing all four samples (Fig 1).
Of the rest, 17 percent (4/24) of the labs scored 3 and failed in the ring test by incorrectly diagnosing one of the four samples; 8 percent of the labs scored 2 and failed in the ring test by incorrectly diagnosing two of the four samples; and 4 percent (1/24) of the labs scored 1 and failed in the ring test by incorrectly diagnosing the results for three of the four samples.
Eleven of the 13 laboratories employing commercial diagnostic kits such as IQ Plus™ WSSV Kit with POCKIT System, IQ 2000 qRT PCR, and other real time PCR kits came up with a correct diagnosis. However, the success rate of correct diagnosis was lower (6 out of 11 or about 54 percent) using conventional nested PCR protocols and other kits.
Perspectives
The aquaculture sector in India has been growing, and for the shrimp farming component, the use of pathogen-free, quality seed has been an important factor in the success and sustainability of shrimp farming; this has been expressed to the stakeholders and farmers by the R&D institutions such CIBA and Govt. bodies such as CAA.
The number of quality, diagnostic services laboratories that can provide quality diagnostic support must increase in the private sector to cater to the needs of the growing shrimp farming sector. The success of shrimp farming depends on the right choice and proper use of diagnostics by aquaculturists to make rapid, prudent and cost-effective management decisions, and where stocking of pathogen free healthy seed is crucial for the successful crop outcome.
Ring tests and harmonization of diagnostic services must be an ongoing and continuous endeavor. At the end of the successful completion of each ring test, CIBA-CAA- and RGCA-MPEDA jointly evaluate the results and notify the private laboratories qualifying to the level of accredited standards. Further, to keep the farmers’ interests, the ICAR-CIBA and the Department of Animal Husbandry, Dairying & Fisheries (DAHD&F), under the MoA&FW approved referral laboratory for brackish water aquaculture for disease diagnostics, shall do the up keeping and validation of the diagnostics services from private laboratories, where farmers can approach the referral lab any time for advice.
Authors
-
Dr. S.V. Alavandi, Ph.D.
ICAR-Central Institute of Brackishwater Aquaculture (CIBA)
Chennai, India -
Dr. Joseph Sahaya Rajan
ICAR-Central Institute of Brackishwater Aquaculture (CIBA)
Chennai, India -
Dr. Biju Narayanan
Rajiv Gandhi Centre For Aquaculture (RGCA)
Sirkali, Tamil Nadu, India
-
Dr. S.K. Otta
ICAR-Central Institute of Brackishwater Aquaculture (CIBA)
Chennai, India -
Dr. M. Poornima
ICAR-Central Institute of Brackishwater Aquaculture (CIBA)
Chennai, India -
Dr. K.K. Vijayan
ICAR-Central Institute of Brackishwater Aquaculture (CIBA)
Chennai, India
Tagged With
Related Posts

Health & Welfare
Double-stranded RNA against WSSV genes provides antiviral protection in shrimp
Silencing genes in white spot syndrome virus (WSSV) with critical roles in replication could provide a strong antiviral effect and thus reduce shrimp mortality. The authors therefore established a study to evaluate the antiviral efficacy of double-stranded (ds)RNA against non-structural WSSV genes.

Health & Welfare
Measuring susceptibility differences to IHHNV infection
This study evaluated the susceptibility to IHHNV in three different shrimp batches by measuring infectivity titers. Each batch showed a different infectivity titer, therefore, each batch had a specific susceptibility to IHHNV.

Health & Welfare
Probiotics benefit Pacific white shrimp challenged with AHPND
A study was conducted to measure the effects of commercial probiotics on Pacific white shrimp in a standardized AHPND challenge model under controlled laboratory conditions. Results show that the probiotics treatments by themselves have beneficial effects, such as higher survival and histological signs of hepatopancreas regeneration.

Aquafeeds
Biofloc consumption by Pacific white shrimp postlarvae
The stable isotopes technique with δ13C and δ15N can be used to determine the relevance of different food sources to shrimp feeding during the pre-nursery phase of Litopenaeus vannamei culture. During this trial, different types of commercial feed, microalgae, Artemia sp. nauplii and bioflocs were used as food sources.


