Study is first to report a ‘lingering phase’ of this important disease

Since 2009, Acute Hepatopancreatic Necrosis Disease (AHPND) progressively spread as an epidemic, devastating shrimp production across much of the shrimp farming regions in Asia and eventually the Western Hemisphere, including an outbreak identified on a farm in the United States.
Previous estimates of the cumulative economic impact of AHPND have ranged from $8 billion (U.S.) for Asia and $4 billion for America. However, in recent years, shrimp production has improved in countries previously hard hit by AHPND, as farmers have tested, improved and adapted husbandry practices that mitigate important environmental risk factors; and as knowledge of the molecular features, detection methods and options for AHPND management have evolved.
The list of bacteria that can cause AHPND include: V. parahaemolyticus (VPAHPND), Vibrio campbellii, V. harveyi, V. owensii and V. punensis. The pathology of AHPND has two distinct phases. In the acute phase, the infected hepatopancreas shows detachment of tubule epithelial cells from the basement membrane and tubule epithelial degeneration in the absence of bacterial cells. In the terminal stage, the hepatopancreas shows extensive intra-tubular hemocytic infiltration and the development of massive secondary bacterial infection.
https://www.aquaculturealliance.org/advocate/four-ahpnd-strains-identified-on-latin-american-shrimp-farms/
Anecdotal evidences suggest that in Latin America, AHPND might be present in several countries including Mexico, and through South America. Even though the Latin American strain of VPAHPND is also highly pathogenic, often VPAHPND does not cause acute mortalities, and actually, in the region, shrimp production has increased steadily during the last 10 years.
This article – summarized from the original publication [L.F. Aranguren Caro et al. 2020. Acute hepatopancreatic necrosis disease (VPAHPND), a chronic disease in shrimp Penaeus vannamei population raised in Latin America. Journal of Invertebrate Pathology, Vol. 174, July 2020, 107424] – reports on VPAHPND challenge trials conducted using Pacific white shrimp (Penaeus vannamei) in Aquaculture Pathology Laboratory (APL) following standard bioassay methodology, as well as assessments carried out to document histopathology and hepatopancreas heterotrophic plate count (HPC). Our results help explain the relative lack of acute mortalities observed at the shrimp farm level in Latin America.
Study setup
Shrimp and inoculum
Latin American lines (All Pathogens Exposed, APE) of P. vannamei were imported into the United States several years ago by a commercial operation. In 2017-2018, three lines of fifth-generation offspring derived from the founding population were submitted to APL for VPAHPND challenge bioassays. Specific-pathogen-free (SPF) P. vannamei (known to be highly susceptible to AHPND) were supplied by a commercial entity in the United States and served as the positive control line in the two trials we conducted.
The reference strain, Vibrio parahaemolyticus, 13-028/A3 (designated VPAHPND), an AHPND causing bacterial strain, was used for both challenge trials. VPAHPND was grown in Tryptic soy broth with 2 percent NaCl (TSB+) at 28 to 29 degrees-C with shaking (120 rpm) for 18 hours or until a bacterial optical density absorbance of 3.0 at OD600 nm (~109 CFU (colony forming units) per ml) was achieved.
Challenge tests
Two VPAHPND challenge trials were carried out in this study. For the exposure tanks (both trials), VPAHPND in TSB was added directly into the water of each challenge tank to achieve an estimated density of 1 × 105 CFU per ml of the bioassay tank water. No water exchange was done after the bacterial inoculation. Mortality was recorded daily for each tank from the start of each experiment.
Trial I
The purpose of Trial I was to document survival for comparison among three shrimp lines, their negative controls and the SPF positive control. Three tank replicates for each of the test lines and two replicate tanks with SPF animals were subjected to immersion challenge, with each tank having 21±3 shrimp. The SPF populations present in the holding tanks were considered as the negative control treatment test for the SPF line in Trial I. All surviving shrimp were fixed at the end of the challenge for histology [microscopic anatomy of biological tissues] examination. The duration of the assay was 11 days.
Trial II
Trial II was carried out to measure hepatopancreas (HP) heterotrophic plate count [HPC; a procedure for estimating the number of live heterotrophic bacteria in hepatopancreas]; histology at 24 hours post-infection (h.p.i); as well as final survival counts and histology at trial termination on six days post infection (d.p.i) To compensate for a possible tank effect, seven shrimp from each line and a similar number of SPF population were stocked into a 1,000-liter tank, while the remaining tagged shrimp from each group were placed into the negative control tank. To identify individual shrimp to group, each animal was injected with a colored (different color for each line), elastomer tag. On day 0, the treatment tank was VPAHPND challenged following the same protocol described for Trial I.
For detailed information on the study design and experimental protocol; Duplex PCR for the detection of pirA- and pirB-like genes; histopathology; and statistical analyses, please refer to the original publication.
Results
Cumulative survival for Trials I and II
Analysis of the survival values using the Kaplan-Meier method revealed significant difference (P<0.001) between APE1 line (72.1±17.4 percent) and the three other lines exposed to VPAHPND (Fig. 1). The APE2 and APE3 lines had similar final survivals of 16.1±5.3 percent and 17.0±10.1 percent, respectively, and the SPF line had the lowest survival (12.5±10.6 percent). Survival was not significantly different (P>0.05) between the APE2, APE3 and SPF lines. For each of the four lines tested, the mean negative control survival (95.2±4.6 percent) was significantly higher (P<0.001) than the mean survival of the corresponding treatment.

PCR
Eleven samples of hepatopancreas from dead shrimp in the VP AHPND Trial I were collected and pooled per line/tank and tested by a duplex PCR [a Polymerase Chain Reaction test that simultaneously detects and differentiates two different genes, pirA and pirB encoding the binary toxin] assay to detect pirA and pirB. All the pooled samples were positive for both pirA and pirB and the bands that amplify the region of 284 bp and 392 bp were clearly observed. Samples from the unexposed lines (negative controls) were pooled per tanks and analyzed by duplex PCR, with negative results in all the cases.
Histopathology
The histopathology of the SPF population in Trial II is presented in Figs. 2 and 3. The Davidson-fixed [a fixative solution to preserve tissue samples] shrimp from the healthy, negative control shrimp in the SPF line displayed normal structure of tubules and epithelial cells in the hepatopancreas, including high levels of lipid droplets (R-cells), secretory vacuoles (B-cells) and absence of AHPND (Fig. 2A). In contrast, SPF shrimp fixed in the first and second day post infection (d.p.i) displayed lesions typical of VPAHPND in the acute phase, including a multifocal necrosis [dead cells in several locations] and massive sloughing of HP tubule epithelial cells in the medial region of the hepatopancreas and progressing outward to the distal region. Very low levels of B-cells and R-cells were observed (G0-G1) and hemocytic infiltration surrounding necrotic tubules was common. At this point, bacterial colonization was not observed (Fig. 2B).

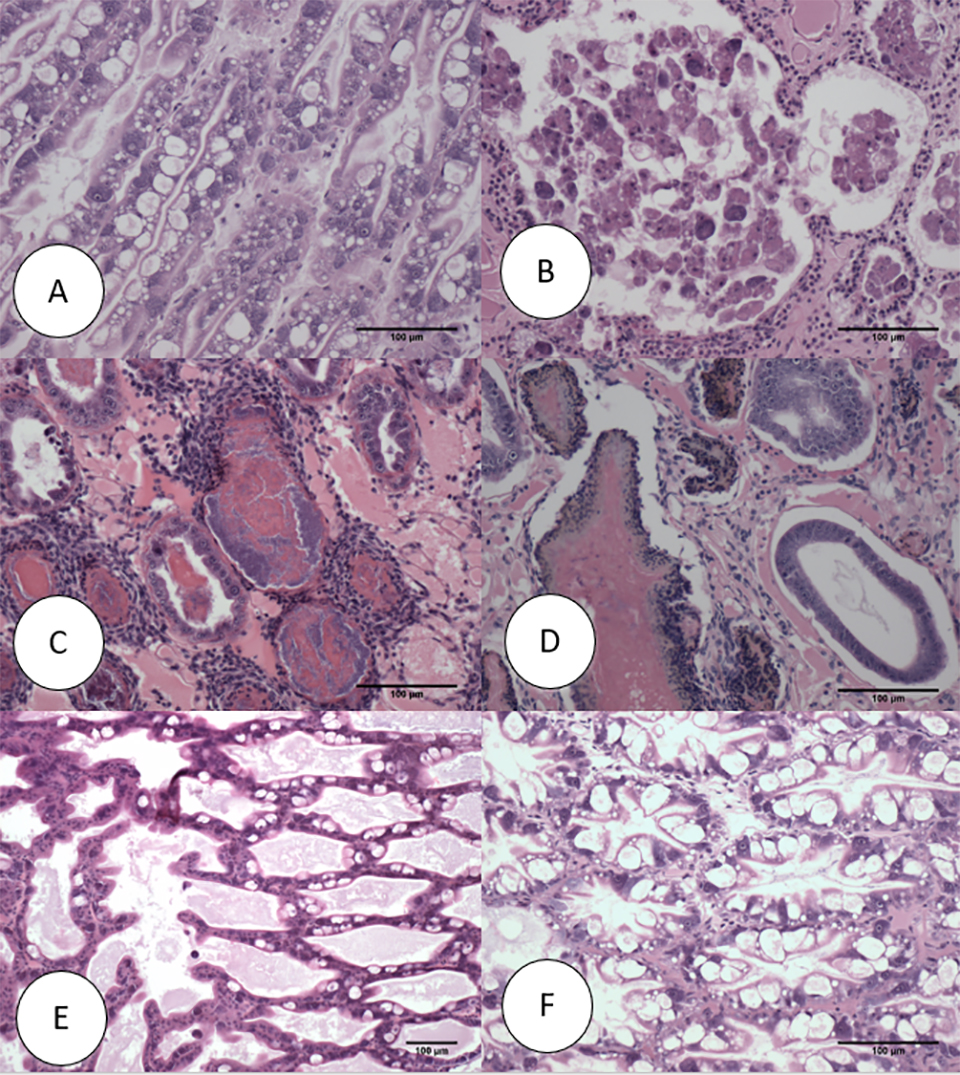
Some of the other SPF shrimp analyzed displayed the typical VP AHPND terminal phase characterized by necrosis and sloughing of HP tubule epithelial cells to (Fig. 2C) massive bacterial presence and host inflammatory response between and within HP tubules. Interestingly, some survivors at 7 d.p.i showed only a few tubules with epithelial necrosis accompanied by bacteria and inflammation, which resembles a septic hepatopancreatic necrosis (SHPN) (Fig. 2D), which has not previously been described as a type of lesion in the case definition for AHPND (Table 1).
Aranguren, LatAm Shrimp AHPND, Table 1
| Tank No. | Shrimp line | No. shrimp | Treatment | Immersion challenge dose of AHPND-VP (CFU/ml) |
|---|
Tank No. | Shrimp line | No. shrimp | Treatment | Immersion challenge dose of AHPND-VP (CFU/ml) |
|---|---|---|---|---|
| A4 | APE2 | 22 | AHPND Negative control | Unexposed |
| A5 | APE3 | 19 | AHPND Negative control | Unexposed |
| A6 | APE1 | 25 | AHPND Negative control | Unexposed |
| L1 | SPF | 20 | AHPND Positive control | 1 × 10^5 |
| L2 | SPF | 20 | AHPND Positive control | 1 × 10^5 |
| D1 | APE2 | 18 | AHPND | 1 × 10^5 |
| D2 | APE2 | 19 | AHPND | 1 × 10^5 |
| D3 | APE2 | 19 | AHPND | 1 × 10^5 |
| D4 | APE3 | 19 | AHPND | 1 × 10^5 |
| D5 | APE3 | 21 | AHPND | 1 × 10^5 |
| D6 | APE3 | 17 | AHPND | 1 × 10^5 |
| 5A | APE1 | 25 | AHPND | 1 × 10^5 |
| 5B | APE1 | 28 | AHPND | 1 × 10^5 |
| 5C | APE1 | 25 | AHPND | 1 × 10^5 |
On the other hand, the histopathology of shrimp representing the Latin American lines (APE1, APE2 and APE3) is presented in Fig. 3. Fig. 3A shows the HP histology of a healthy shrimp from the APE negative control tank. The histology was similar to the normal HP histology in the animals from the SPF line. Figs. 3B and 3C, respectively, are histology examples of the typical acute and terminal phase of AHPND and Fig. 3D is an example of the appearance of chronic phase AHPND histology for a survivor shrimp after 6 d.p.i. Similar chronic, histopathology lesions were observed in shrimp in the SPF line. The granulomatous response in the HP tubules would be an indicator of an inflammatory response at a more advanced stage of maturity in the chronic phase in AHPND-affected shrimp. Unlike the previous set of figures, Fig. 3E presents a suspected case of AHPND (G-trace) with focal necrosis and sloughing of tubules epithelial cells in the medial portion of HP. Two survivors from APE1 had no histopathological lesions indicative of AHPND, which suggest a possible tolerance/resistance to AHPND infection (Fig. 3F).
Bacteriology
Fig. 4 summarizes the HPC for the HP of control and VP AHPND challenged shrimp at 24 h.p.i. For the negative control group, the highest mean count was recorded for APE1 (2.22 × 107 CFU per gram HP). In contrast, the lowest mean control count was for the SPF line (1.05 × 104 CFU per gram HP). The 24 h.p.i. VPAHPND challenge tank samples showed the HPC increased significantly (P<0.05) between control and treatment shrimp for the APE2, APE3 and SPF lines, with the largest differential mean count: 3.70 × 107 CFU per gram HP for the SPF line, ~103 times higher than the mean negative control count for this line (1.05 × 104 CFU per gram HP). The APE1 line had little change in mean CFU per gram HP between the negative control (2.20 × 107 CFU per gram HP) and the VP AHPND challenged cohort samples (2.13 × 107 CFU per gram HP) in the study.

Discussion
In this study, four P. vannamei shrimp lines, including an SPF line and three Latin American shrimp lines, were challenged via immersion with a virulent strain of VPAHPND using a bacterial cell density of ~1×105 CFU per ml. One of the Latin American lines (APE1 line) displayed significantly improved survival as compared to the APE2, APE3 and the SPF lines to VPAHPND challenge under laboratory experimental conditions examined.
AHPND acute and terminal phase lesions were recorded in moribund shrimp in the two trials. In Trial I, the survivor shrimp (6 d.p.i) also showed a histology change and septic hepatopancreatic necrosis (SHPN) (Figs. 2D, 3C & 3D). It is interesting to note that SHPN was not included in the pathology description of AHPND (Lightner & Flegel 2012, OIE 2018)probably because during the first years of the infection in Southeast Asia, the mortality was close to a 100 percent in most of the cases.
To our knowledge, this is the first record of SHPN in VPAHPND infected shrimp. Other chronic, hepatopancreas changes observed were granulomata, focal to multifocal melanization of HP tubules, low cytoplasmic lipid and atrophy of tubule epithelial cells. One survivor from the APE group had mild tubule epithelial necrosis (Fig. 3E) and in another specimen no AHPND lesions were observed at all (Fig 3F). These histological features could indicate tolerance/resistance in shrimp from APE 1 line.
In Trial II, the HPC in hepatopancreas increased at 24 h.p.i. in the VPAHPND treatments when compared to the negative control tank for the APE2, APE3 and the SPF lines. The CFU per gram HP control counts in the APE2, APE3 and SPF lines ranged between ~104 and ~106; whereas for the APE1 line the CFU per gram HP were significantly higher (mean = 2.20 × 107 grams HP). The highest control to challenge differential was 1: 3.01 × 103 for the SPF line; whereas for the high survival APE1 line, the mean difference was 1.04:1, essentially unchanged between the control and the VPAHPND challenged shrimp.
Following the VPAHPND challenge, the mean CFU per gram HP increased for lines 2, 3 and 4. The mean HPC was 1.7 × 107 CFU per gram HP (range: 9.52 × 104 to 1.05 × 108 CFU per gram HP). Although the bacterial load was quite variable among different animals, the CFU mean was consistent with previous challenge test studies we have carried out at the APL. In our data, the hepatopancreas bacterial count which was comprised of the VPAHPND as well as the natural bacterial flora likely increased in response to the increased availability of nutrients (necrotic cellular debris and exposed tubule basal membrane) in the affected HPs.
In this study, improved survival was associated with very high hepatopancreas HPC prior to the VPAHPND challenge in the APE1 line. Perhaps an initial high bacteria biomass in the stomach/hepatopancreas had a negative influence on V. parahaemolyticus colonization and/or replication, either through direct bacteria to bacteria antagonism and/or the high bacteria load was associated with greater activity of the shrimps’ innate immune system, thus giving the individual APE1 animals an increased host tolerance to AHPND. In any case, the reason(s) behind the higher survival recorded for APE1 would be worthwhile to explore further.
Despite the strong evidence of the presence of AHPND in Latin America, farmed shrimp production in this region has grown continuously during the last 10 years (GOAL 2019, FAO 2018). The chronic mortalities caused by AHPND at the farm level in grow-out ponds were similar to the chronic mortalities reported in our study through a VPAHPND challenge test of the shrimp lines used in Latin America. In order to explain this AHPND resistance/tolerance, it is important to look at the history of the shrimp industry in Latin America. Since the early days, the vast majorities of farms in Latin America have utilized a “live with the pathogens” approach, referred to as All Pathogens Exposed (APE).
The shrimp industry in Latin American countries has been exposed to several pathogens during the last 30 years, including viral pathogens such as Taura Syndrome Virus (TSV) from 1992 to 1995 and White Spot Syndrome Virus (WSSV) since 1999. With no other options, some of the major producers initiated a program to select the survivors, initially from TSV-infected ponds and later on from WSSV-infected ponds.
In the mid-term, the shrimp industry started seeing some recovery in their ponds previously hit by these viral pathogens. From 2010-2019, the shrimp industry in Ecuador – by far the largest shrimp producer in the Americas – enjoyed increased pond survival and production despite the presence of these pathogens in the culture ponds.
During the TSV and WSSV selection process by the shrimp farmers, some other diseases including bacterial diseases have been present at the farm level – including SHPN and seagull syndrome – and some at the hatchery level (including bioluminescent V. harveyi and Bolitas syndrome), which might suggest that these APE populations have been exposed to several bacterial pathogens and have acquired some tolerance to multiple pathogens simultaneously. Also, shrimp in Colombia selected for resistance to TSV indirectly acquired resistance to Necrotizing Hepatopancreatitis (NHP), an endemic disease present in the broodstock ponds at that time.

We believe that similar developments could have occurred with these Latin American stocks that encountered Vibrio infections during the farm culture conditions. In Latin America shrimp farms, AHPND does not lead to the acute mortalities that occur in Southeast Asia farms. Our study is the first report of a new phase of infection of AHPND, a chronic phase based on experimental AHPND-challenge trials we conducted using shrimp lines from Latin America. Our results explain in part why the shrimp industry in various Latin American countries have continue to expand regardless of the presence of AHPND. In addition, our results show that the biology and pathology of AHPND resistant/tolerant shrimp appear to be quite unique in this Latin American shrimp population.
Perspectives
In summary, we have described bioassay challenge results that identified one of three Latin American lines that displayed improved (~70 percent) survival to AHPND challenge as compared to the two other APE and the SPF positive control lines.
The difference in survival could be attributed to either elevated bacteria recorded in the HP of individual APE1 animals and/or yet unknown attribute to tolerance to AHPND in this line when compared to the three other lines evaluated in the two trials.
Additional comparative studies are planned to learn more about qualitative and quantitative bacteriology in hepatopancreas samples collected in Trial II.
References available from the corresponding author.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-

Luis Fernando Aranguren Caro, Ph.D.
Corresponding author
School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E. Lowell Street
Tucson, Arizona 85721 USA -

Hung N. Mai, Ph.D.
School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E. Lowell Street
Tucson, Arizona 85721 USA -

Brenda Noble, B.Sc.
School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E. Lowell Street
Tucson, Arizona 85721 USA -

Arun K. Dhar, Ph.D.
School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E. Lowell Street
Tucson, Arizona 85721 USA
Tagged With
Related Posts

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Health & Welfare
White Feces Syndrome in shrimp: Predictor of EHP?
Study demonstrates strong association between White Feces Syndrome and Enterocytozoon hepatopenaei in EHP-endemic regions. Biosecurity strategies can minimize the risk of pathogen’s spread in the Americas.

Health & Welfare
EHP a risk factor for other shrimp diseases
Laboratory challenges and a case-control study were used to determine the effects of EHP infection on two Vibrio diseases: acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN).

Health & Welfare
Development of new and real-time PCR for detection and quantification of NHPB
Authors describe conventional PCR and real-time PCR assays based on the NHPB flgE gene as alternative methods for the detection and quantification of NHPB in shrimp and shrimp-associated samples, including artemia. This newly described method will be an additional diagnostic tool for confirmation of this pathogen.

