Results show shrimp meat not infectious after boiling for over 1 minute

White spot disease (WSD), caused by the white spot syndrome virus (WSSV), is one of the biggest threats to the farmed shrimp industry. WSD causes high mortalities that can reach up to 100 percent in Specific Pathogen Free (SPF) populations in grow-out conditions in a matter of days. WSSV was first reported in Taiwan in 1992. Since then, shrimp infections with WSSV have been reported in Japan, Korea, South-East Asia, South Asia, the Mediterranean, the Middle East, the Americas and Australia.
The risk of WSSV introduction is predominantly due to the international movement between producing and importing countries of infected shrimp, whether they are live or frozen – head on (HO), head on shell on (HOSO) or headless shell on (HLSO) – or as cooked products. Due to the risk of inadvertently introducing shrimp pathogens via commodity products, some countries – including Australia, the Kingdom of Saudi Arabia and others – have implemented strict protocols regulating the importation of raw shrimp and prawn products. The screening to determine the presence and/or absence of some viral etiologies is through polymerase chain reaction (PCR). Usually, if the batch is positive, the product is either destroyed or treated (cooked).
Recently, cooked shrimp infected with WSSV tested positive by polymerase chain reaction (PCR). However, there is no study to determine the infectivity of WSSV in cooked shrimp that tested positive by PCR. There have been published studies that indicated that frozen shrimp products were an avenue for WSSV introduction into the Americas in 1995, but there are only a handful of peer-reviewed publications highlighting the risk of WSSV infectivity of cooked commodity shrimp. However, these studies restricted their findings to WSSV detection by PCR and it remained unknown if cooked shrimp that contain DNA viral sequences are infectious. The purpose of our study – supported by the Aquaculture Pathology Laboratory at the University of Arizona – was to evaluate the infectivity of WSSV-infected shrimp upon cooking.
Study setup
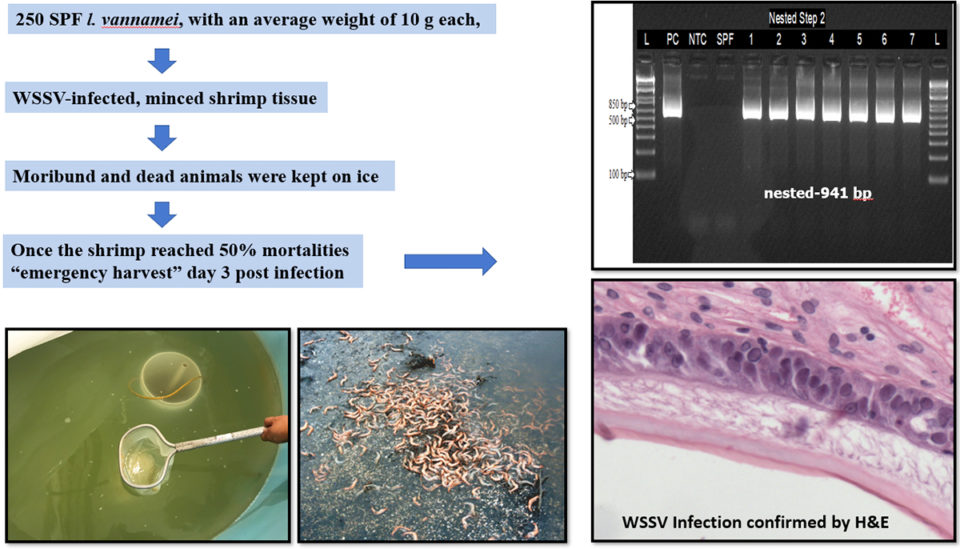
The shrimp in the study came from a commercial shrimp facility in the United States. A total of 250 SPF Pacific white shrimp (Litopenaeus vannamei) with an average weight of 10 grams were used. This sample weight represents the typical weight of shrimp in ponds when emergency harvests are carried out at shrimp farms where WSSV is endemic and a disease outbreak is about to occur.
To produce WSSV-infected shrimp, a WSSV challenge test was conducted via oral route by feeding previously confirmed WSSV-infected, minced shrimp tissue to SPF L. vannamei. This was done at 10 percent body weight of the SPF animals divided into two rations (morning and evening) in one day. Water in the experimental tanks was kept at 25 ppt salinity and a temperature of 25 degrees-C. After the experimental challenge, mortalities were assessed every hour. SPF L. vannamei were fed healthy tissue and served as a negative control.
Once shrimp mortalities reached 50 percent, the surviving animals were removed to simulate an emergency harvest and euthanized. Gills and pleopods samples were collected and preserved for confirmation with conventional and real-time PCR assays. Five shrimp with clinical signs were fixed and preserved for histological analysis (Fig. 1).
The initial infected shrimp population was separated into six groups each with 40-shrimp; each group was assigned to a specific boiling (in freshwater) time of 0, 1, 3, 5, 10 and 30 minutes. One group, not infected with WSSV, was the negative control. Each 40-shrimp group was divided into two sub-groups with two replicates each. Sub-group 1 was boiled using the different time frames (0, 1, 3, 5, 10 and 30 minutes) before feeding to SPF L. vannamei. Sub-group 2 was also boiled for different time frames (0, 1, 3, 5, 10 and 30 minutes) but was frozen (minus-20 degrees-C) for 14 days before being fed to SPF shrimp (Fig. 2).
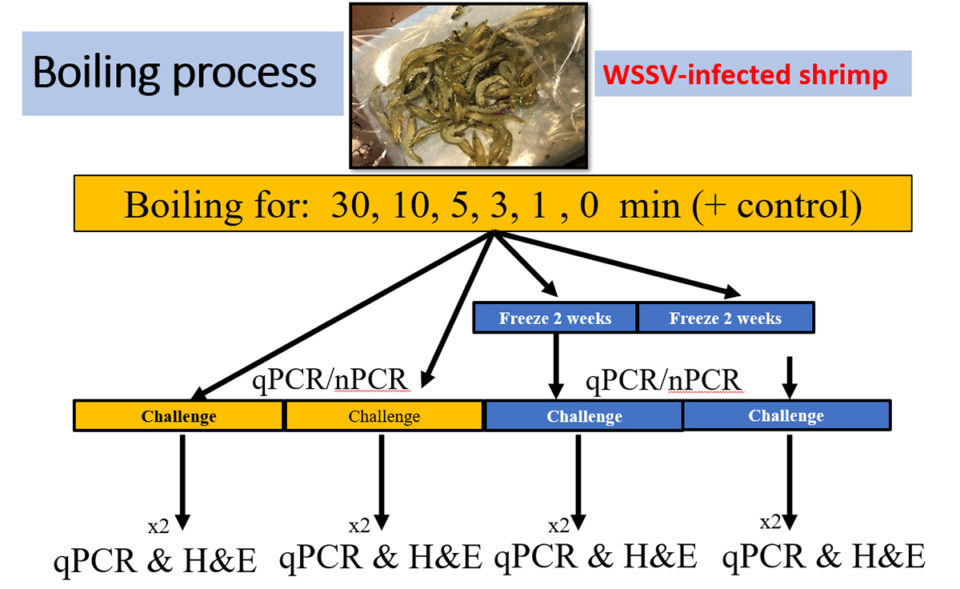
Temperature in shrimp tissue were collected using a thermometer placed inside the tail skeletal muscle, and internal temperature data were collected before and after the cooking process. After the boiling, samples were allowed to cool down slightly and then gill and pleopod samples were collected and preserved for further analysis of both PCR and qPCR (quantitative PCR is an improved version of traditional PCR and is used to detect and quantify nucleic acids for numerous applications).
To determine the pathogenicity of the cooked shrimp, a challenge test was conducted with a SPF shrimp population. Each 90-liter tank contained 10 juveniles (2 to 3 grams). Out of each replicate from each time frame, shrimp tissues including stomach, gills and pleopods were chopped from all the shrimp and minced. The minced tissue was added to the tank in two rations (morning and evening) at 10 percent of the total body weight of the live SPF population in one day. The duration of the challenge was 11 to 12 days. Daily mortalities were recorded, and pools of gills/pleopods from moribund shrimp were collected and preserved to be used for WSSV detection by qPCR and PCR. In addition, the moribund shrimps were fixed in Davidson’s fixative for histological analysis.
The final cumulative survival was determined at the end of the challenge. Samples of pleopods of all the shrimp were pooled per tank and preserved in 95 percent ethanol for qPCR and PCR analysis. Two shrimp from each tank were fixed in Davidson’s fixative for histological analysis. Shrimp frozen for two weeks (Group 2) were used to conduct a similar WSSV infection challenge using the same methodology described above.
For detailed information on the experimental design; shrimp experimental infection, sampling and pooling, and boiling of samples; the WSSV-challenge test; DNA extraction; PCR and qPCR analyses; and histopathology, contact the corresponding author.
Results
The WSSV-infected shrimp tissue added to the experimental tank with a shrimp population of 10.5 grams ± 1 gram caused high mortalities. The first group of shrimp began dying on day 2 post-infection (d.p.i). On 4 d.p.i., when the mortality reached 50 percent, survivors were harvested. Davidson’s-fixed shrimp were confirmed to be positive for WSSV by PCR and H&E (haematoxylin and eosin stain, or H&E stain, is one of the principal tissue stains used in histology, the study of the microscopic anatomy of biological tissues; see Fig. 1). Thirty shrimp collected during the challenge were maintained on ice, then sampled and confirmed positive for WSSV by qPCR and nested PCR (a modification of PCR designed to improve specificity and sensitivity).
The WSSV-infected shrimp were exposed to different boiling temperature (0, 1, 3, 5, 10 and 30 minutes). At the end of the boiling process, samples of gills and pleopods were taken for PCR and qPCR analysis. Table 1 summarizes the qPCR and nested PCR results for WSSV without freezing and frozen for two weeks. Samples collected upon cooking for different time provided gave positive results for WSSV by qPCR. In contrast, none of the samples provided any amplification by nested PCR.
Aranguren, cooked shrimp, Table 1a
| Tank description (without freezing) | qPCR WSSV – No. positive/No. total | Ct Value – Mean ± SD | Nested PCR WSSV – No. positive pools/No. total |
|---|---|---|---|
| Negative control | 0/4 | Not detected | ND (0/1) |
| WSSV 30 min cook | 4/4 | 21.48±3.05 | ND (0/1) |
| WSSV 10 min cook | 4/4 | 18.44± 0.61 | ND (0/1) |
| WSSV 5 min cook | 4/4 | 20.54 ±3.20 | ND (0/1) |
| WSSV 3 min cook | 4/4 | 20.01± 1.81 | ND (0/1) |
| WSSV 1 min cook | 4/4 | 25.73± 5.00 | ND (0/1) |
| WSSV 0 min cook (Positive control) | 4/4 | 20.35± 2.50 | Positive (1/1) |
Aranguren, cooked shrimp, Table 1b
| Tank Description (freezing for 2 weeks) | qPCR WSSV – No. positive/No. total | Ct Value – Mean ± SD | Nested PCR WSSV – No. positive pools/No. total |
|---|---|---|---|
| Negative control | 0/4 | Not detected | ND (0/1) |
| WSSV 30 min cook | 4/4 | 21.10±2.29 | ND (0/1) |
| WSSV 10 min cook | 4/4 | 21.22±1.30 | ND (0/1) |
| WSSV 5 min cook | 4/4 | 22.99±2.15 | ND (0/1) |
| WSSV 3 min cook | 4/4 | 22.16±2.06 | ND (0/1) |
| WSSV 1 min cook | 4/4 | 20.65±1.03 | ND (0/1) |
| WSSV 0 min cook (Positive control) | 4/4 | 21.34±2.63 | Positive (1/1) |
In treatment Group 1, shrimp tissue infected by WSSV and exposed to different boiling times were used as inoculum in a per os challenge test using SPF L. vannamei shrimp. Table 2 summarizes the survival percentage of shrimp after 10 d.p.i.
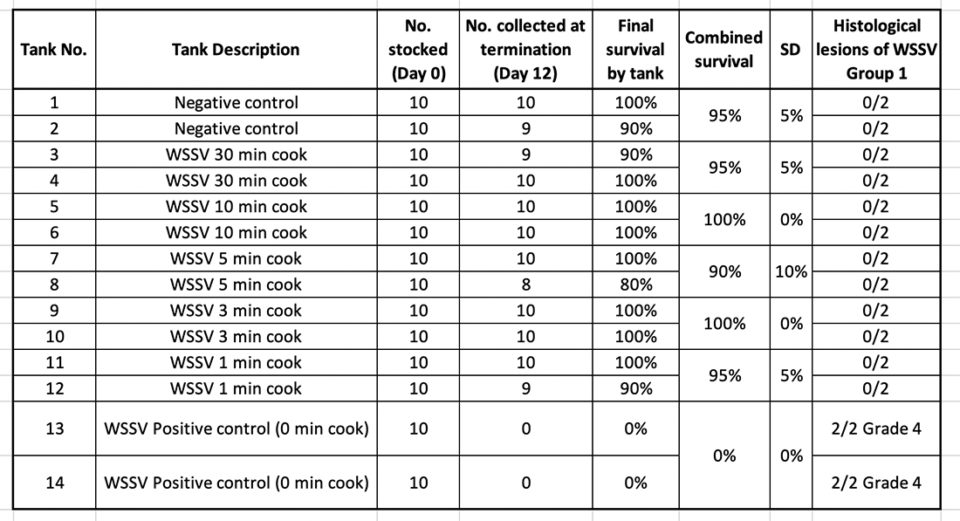
Only high mortality was found in the WSSV positive control (0-minute cook) tanks. H&E and PCR confirmed the presence of WSSV in those tanks. Similar results were obtained when the boiled shrimp were frozen for two weeks and then used as a source of infectious material.
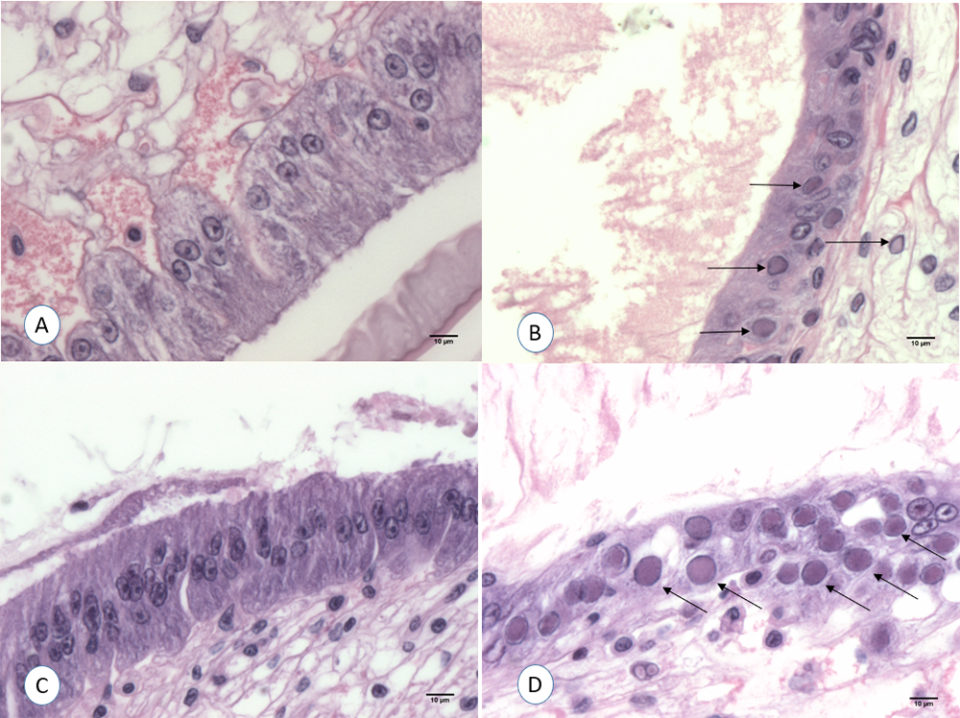
The samples collected for H&E from the different treatments at the end of the experiment were analyzed. WSSV intranuclear basophilic inclusion bodies within the hypertrophied nuclei were found in several affected tissues of ectodermal and mesodermal origin. Fig. 3 shows the typical WSSV histological lesions Grade 4.0 in stomach cuticular epithelia from the positive control tank. In contrast, none of the shrimp fed with shrimp tissue upon boiling for different times (1, 3, 5, 10 and 30 minutes) showed any histological lesions typical of WSSV.
The samples of the juveniles experimentally infected with boiled shrimp that tested positive by qPCR for WSSV were negative in all the cases (Table 3).
Aranguren, cooked shrimp, Table 3
| Tank description | WSSV-infected shrimp cooked at different times | qPCR WSSV – No. positive/No. total | Ct Value – Mean ± SD |
|---|---|---|---|
| SPF shrimp challenge with SPF shrimp | Negative control | 0/4 | ND |
| SPF shrimp challenge with WSSV infected shrimp cooked for 30 min | SPF challenge with WSSV 30 min cook-shrimp | 0/4 | ND |
| SPF shrimp challenge with WSSV infected shrimp cooked for 10 min | WSSV 10 min cook | 0/4 | ND |
| SPF shrimp challenge with WSSV infected shrimp cooked for 5 min | WSSV 5 min cook | 0/4 | ND |
| SPF shrimp challenge with WSSV infected shrimp cooked for 3 min | WSSV 3 min cook | 0/4 | ND |
| SPF shrimp challenge with WSSV infected shrimp cooked for 1 min | WSSV 1 min cook | 0/4 | ND |
| SPF shrimp challenge with WSSV infected shrimp cooked for 0 min | WSSV positive control (0 min. cook) | 4/4 | 21.57±1.73 |
Discussion
Our results proved that WSSV-infected shrimp exposed to boiling temperature for times that ranged from 1 to 30 minutes were not infectious. The qPCR data confirmed the presence of WSSV DNA in all samples exposed to different boiling times, and no significant difference of the Ct (cycle threshold, the cycle number when the fluorescence of a PCR product can be detected above the background signal) values were found among the different boiling times (P<0.05). However, none of the samples provided any successful amplification by nested PCR WSSV.
The qPCR results showed positive results, which contradicted the negative results with nested PCR. It is likely that WSSV DNA isolated from boiled shrimp had undergone significant damage resulting in reduced quality and fragmented than those isolated from a freshly harvested sample. The expected size of nested PCR step-1 amplicon (a piece of DNA that is the product of amplification or replication events) is 1,447 bp, and the internal step-2 amplicon is 941 bp. The absence of the 1,447-bp and 941-bp amplicons in the nested PCR results shows that WSSV DNA has been degraded in smaller fragments and therefore lost infectivity. In contrast, the qPCR amplicon size is only 69-bp, a significantly smaller amplicon that the nested PCR. As a result, even when the WSSV DNA got fragmented upon boiling, small amplicons can still be amplified and positive results were obtained by qPCR.
In order to confirm the infectivity of the WSSV-infected L. vannamei that tested positive only by qPCR, an experimental challenge test using SPF shrimp was conducted. The final survival was high in all the tanks fed with the qPCR WSSV-positive minced tissue exposed to boiling temperature from 1 to 30 minutes. Mortalities were obtained only in shrimp exposed to the positive control minced tissue (0 minutes boiling). Surviving shrimp at the end of the challenge from all the tanks were analyzed by H&E and qPCR. WSSV inclusion bodies in the cuticular epithelium and connective tissues were found in shrimp that had been fed with the positive control minced tissue. In contrast, histology of shrimp challenged with WSSV positive tissue boiled at 1, 3, 5, 10 and 30 minutes did not show any histological lesions pathognomonic of WSSV infection. Fig. 3A and 3C show the histological stomach section of a shrimp exposed to WSSV positive tissue boiled for 1 minute. The qPCR analysis was conducted from two pools of each tank. The only qPCR positive results were found in shrimp challenged with WSSV positive tissue (positive control).
The results of our study indicate that cooking shrimp could reduce the spread of WSSV from global exportation/importation routes for farmed shrimp. We believe it would be of interest to the shrimp industry and others to utilize our approach for testing for other microbial pathogens infecting farmed shrimp, including other viruses as well as bacteria and fungi.
Perspectives
In our study, WSSV-infected shrimp were cooked at boiling temperature for different times, including 0, 1, 3, 5, 10 and 30 minutes. After exposure to boiling temperature, the WSSV-infected shrimp were fed to SPF Pacific white shrimp (L. vannamei). The results showed that experimentally challenged shrimp from the 0-minute treatment (positive control) indeed got infected with WSSV. However, experimentally challenged shrimp that were fed tissues boiled for 1, 3, 5, 10 and 30-minute were not infected with WSSV.
The resulting mortality data showed that only the positive control (0-minute) treatment displayed high mortality, whereas no mortalities were observed in any of the other treatments. These findings suggest that cooking shrimp at boiling temperature for at least 1 minute might prevent any potential spread of WSSV from endemic countries to other geographical areas where WSSV has not yet been reported. A nested method for WSSV detection should be used instead of the qPCR method while assessing infectivity of cooked shrimp.
References available from the corresponding author.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-

Luis Fernando Aranguren Caro, Ph.D.
Corresponding author
School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E. Lowell Street
Tucson, Arizona 85721 USA[117,100,101,46,97,110,111,122,105,114,97,46,108,105,97,109,101,64,117,103,110,97,114,97,102,108]
-

Hung N. Mai, Ph.D.
School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E. Lowell Street
Tucson, Arizona 85721 USA -
Linda Nunan, MSc
School of Animal and Comparative Biomedical Sciences
University of Arizona, 1117 E. Lowell Street
Tucson, Arizona 85721 USA -
Joshua Lin, BSc
School of Animal and Comparative Biomedical Sciences
University of Arizona
1117 E. Lowell Street
Tucson, Arizona 85721 USA -

Brenda Noble, BSc
School of Animal and Comparative Biomedical Sciences
University of Arizona
1117 E. Lowell Street
Tucson, Arizona 85721 USA
-

Arun K. Dhar, Ph.D.
School of Animal and Comparative Biomedical Sciences
University of Arizona
1117 E. Lowell Street
Tucson, Arizona 85721 USA
Tagged With
Related Posts

Health & Welfare
The history and future of the Aquaculture Pathology Laboratory
The University of Arizona’s Aquaculture Pathology Laboratory has significantly contributed to the expansion of the shrimp farming for three decades.

Health & Welfare
The unmet promise of pondside PCR
A new generation of technology, portable PCR, offers potential for affordable, immediate, pondside diagnosis in easy-to-use handheld kits. But will it live up to the hype?

Health & Welfare
Biosecurity practices on fish farms need beefing up
Biosecurity measures and preventive strategies are essential in any biological production chain. Properly planned and implemented biosecurity programs will enhance animal health, production and economics.

Health & Welfare
CENIACUA develops WSSV-resistant shrimp in Colombia
To combat white spot syndrome virus (WSSV) in white shrimp, Corporación Centro de Investigación de la Acuacultura de Colombia (CENIACUA) initiated a selective-breeding program to develop resistance in shrimp.


